Gut-eye axis
- PMID: 40567942
- PMCID: PMC12192331
- DOI: 10.1016/j.aopr.2025.01.003
Gut-eye axis
Abstract
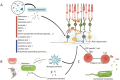
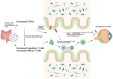
Background: The gut microbiome, colonizing the human gastrointestinal tract, is increasingly recognized for its symbiotic relationship with the immune system in maintaining overall host health. This emerging understanding raises intriguing questions about potential connections between the gut microbiome and anatomically distant organs, such as the eye, possibly mediated through immune pathways.
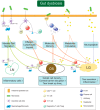
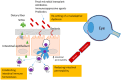
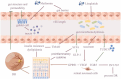
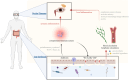
Main text: This review synthesizes contemporary research on ocular diseases with the framework of the burgeoning "gut-eye axis" concept. Investigations spanning from the ocular surface to the fundus suggest correlations between the gut microbiome and various ocular disorders. By elucidating the putative pathogenic mechanisms underlying these ocular conditions, we offer novel perspectives to inform future diagnostic and therapeutic interventions in ophthalmology.
Conclusions: By presenting a critical analysis of current knowledge regarding the role of gastrointestinal microbiota in ocular health, this review shed light on the complex interplay between gut dysbiosis and eye disorders. Our work endeavors to catalyze interdisciplinary research and foster innovative clinical applications, thereby bridging the gap between the gut microbiota and the ocular well-being.
Keywords: Gut microbiota; Gut-eye axis; Immune dysregulation; Microbial-derived metabolites; Ocular disease; The leaky gut.
© 2025 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

