Pharmacokinetics of Subcutaneous and Intravenous Ceftriaxone in an Older Population: The PhASAge Study
- PMID: 40567999
- PMCID: PMC12188194
- DOI: 10.1093/ofid/ofaf313
Pharmacokinetics of Subcutaneous and Intravenous Ceftriaxone in an Older Population: The PhASAge Study
Abstract
Background: Ceftriaxone is frequently administered subcutaneously in France, especially in older patients, although this practice is currently off-label. This work aims to describe the pharmacokinetics (PK) and pharmacodynamics (PD) and tolerance of ceftriaxone administered by intravenous and subcutaneous routes in older patients.
Methods: Patients aged ≥65 years receiving intravenous or subcutaneous ceftriaxone 1 g every 24 hours were included. Steady-state plasma concentrations of ceftriaxone were measured. Based on intravenous and subcutaneous ceftriaxone concentrations and 24-hour area under the concentration-time curve (AUC), a population PK model was developed for probability of target attainment (PTA) analysis. Local and systemic adverse events (AEs) were collected.
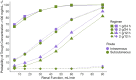
Results: Data from 47 patients (24 in subcutaneous and 23 in intravenous groups) were analyzed. No between-group difference was observed in demographic and biological characteristics, ceftriaxone trough concentrations, or AUC. Bioavailability of subcutaneous ceftriaxone was estimated at 99% by population modeling. The PTA associated with subcutaneous administration were similar to or slightly better than that of the intravenous route. A dosing regimen of 1 or 2 g every 24 hours was associated with acceptable PTA and a low risk of overexposure in patients with normal or moderately altered renal function. Tolerance was assessed on 149 infusions (67 intravenous and 82 subcutaneous). One local AE (1.5%) was reported in the intravenous group versus 11 local AEs (mainly edema) in the subcutaneous group (13%), all transient and nonsevere.
Conclusions: Subcutaneous administration of ceftriaxone was associated with PK/PD and dosage requirements comparable to those of intravenous administration, supporting the use of subcutaneous ceftriaxone in older patients.
Keywords: ceftriaxone; older adults; pharmacokinetics; subcutaneous; tolerance.
© The Author(s) 2025. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. All authors: No reported conflicts.
Figures

References
-
- Richards DM, Heel RC, Brogden RN, Speight TM, Avery GS. Ceftriaxone: a review of its antibacterial activity, pharmacological properties and therapeutic use. Drugs 1984; 27:469–527. - PubMed
-
- European Medicines Agency . Pursuant to article 30 of directive 2001/83/EC—Rocephin and associated names. 2014. Report EMA/144854/2014. Available at: https://www.ema.europa.eu/en/documents/referral/rocephin-article-30-refe.... Accessed 15 October 2022.
-
- Forestier E, Paccalin M, Roubaud-Baudron C, Fraisse T, Gavazzi G, Gaillat J. Subcutaneously administered antibiotics: a national survey of current practice from the French Infectious Diseases (SPILF) and Geriatric Medicine (SFGG) society networks. Clin Microbiol Infect 2015; 21:370.e1–e3. - PubMed
-
- Mesples LM, Forestier E, Paccalin M, et al. O-108 European survey on subcutaneous antibiotics administration. Presented at: 19th European Geriatric Medicine Society Congress; Helsinki. 2023.
-
- Forestier E, Janosh L, Vitrat V, et al. Subcutaneous antibiotic therapy use by French general practitioners: its interest and limitations. Infect Dis Now 2023; 53:104768. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

