This is a preprint.
Immune Niche Formation in Engineered Mouse Models Reveals Mechanisms of Tumor Dormancy
- PMID: 40568095
- PMCID: PMC12190759
- DOI: 10.1101/2025.04.16.649000
Immune Niche Formation in Engineered Mouse Models Reveals Mechanisms of Tumor Dormancy
Abstract
Residual tumor cells can persist in a dormant state during clinical remissions that may last decades. The mechanisms that lead to such growth control vs. eventual reactivation and macroscopic tumor outgrowth remain unclear. Here, we report data from a mouse model that reveals a key role of host immunity and the cellular and molecular mechanisms that control tumor dormancy. Abrogation of myeloid-specific TGF-βRII expression (TβRIImyeKO) resulted in an IFN-γ rich immune microenvironment. IFN-γ in turn elevated KLF4-mediated SLURP1 production in malignant cells, which is critical to the tumor cell quiescent state through interruption of fibronectin-integrin signaling pathways. The dormant tumor lesions were located in spatially localized immune niches rich in NK cells, cDCs, monocytes, and neutrophils, concomitant with tumor cell inactivation of NK cell immune surveillance through a CD200-CD200R1 mechanism. Our studies identify the IFN-γ-KLF4-SLURP1 and CD200-CD200R1 axes as critical molecular drivers in tumor dormancy regulated by immune-tumor crosstalk. These insights provide enhanced mechanistic understanding of tumor dormancy in a mouse model suitable for further investigation of cancer treatment resistance and prevention of metastatic spread.
Keywords: TGF-β; Tumor dormancy; imaging; immune; metastasis; myeloid; niche.
Conflict of interest statement
Conflict of interest statement: The authors declare no potential conflicts of interest
Figures
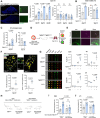
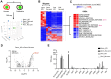





References
-
- Goddard E.T., Bozic I., Riddell S.R. & Ghajar C.M. Dormant tumour cells, their niches and the influence of immunity. Nat Cell Biol 20, 1240–1249 (2018). - PubMed
Publication types
LinkOut - more resources
Full Text Sources
