This is a preprint.
β-III tubulin identifies anti-fibrotic state of pericytes in pulmonary fibrosis
- PMID: 40568101
- PMCID: PMC12190747
- DOI: 10.1101/2025.04.15.648984
β-III tubulin identifies anti-fibrotic state of pericytes in pulmonary fibrosis
Abstract
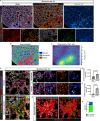
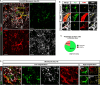
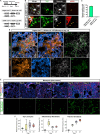
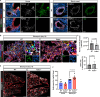
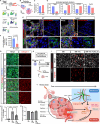
Pericytes have been implicated in pulmonary fibrosis, yet their activated cellular states and functional roles remain largely unclear. Here, we identified β-III tubulin (Tuj1) as a distinctive marker for fibrosis-associated lung pericytes. Most pericytes in fibrotic regions are Tuj1-positive and interact uniquely with multiple endothelial cells, localizing near collagen-producing fibroblasts and pro-fibrotic SPP1+/arginase+ macrophages. Tuj1 expression is predominantly induced in pericytes during the fibrotic phase, and Tuj1 gene (Tubb3) knockout in mice exacerbates lung fibrosis, accompanied by an increase in the neighboring pro-fibrotic fibroblasts and macrophages, suggesting an anti-fibrotic role for Tuj1-expressing pericytes. Mechanistically, the anti-fibrotic chemokine CXCL10 is upregulated in Tuj1-expressing pericytes, whereas this upregulation is not observed in Tubb3 knockout. Moreover, CXCL10 inhibits the pro-fibrotic differentiation of macrophages induced by lung fibroblasts in culture, implying that CXCL10 may mediate the anti-fibrotic effects of Tuj1-expressing pericytes. These findings underscore the role of lung pericytes in negatively regulating fibrotic process and their potential as therapeutic targets for pulmonary fibrosis patients.
Figures





References
-
- Abe Y., Kwon S., Oishi M., Unekawa M., Takata N., Seki F., Koyama R., Abe M., Sakimura K., Masamoto K., Tomita Y., Okano H., Mushiake H., and Tanaka K.F.. 2021. Optical manipulation of local cerebral blood flow in the deep brain of freely moving mice. Cell Rep. 36:109427. - PubMed
-
- Alquicira-Hernandez J., and Powell J.E.. 2021. Nebulosa recovers single-cell gene expression signals by kernel density estimation. Bioinformatics. 37:2485–2487. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
