This is a preprint.
Maintenance of chronic neuroinflammation in multiple sclerosis via interferon signaling and CD8 T cell-mediated cytotoxicity
- PMID: 40568143
- PMCID: PMC12190403
- DOI: 10.1101/2025.06.09.658729
Maintenance of chronic neuroinflammation in multiple sclerosis via interferon signaling and CD8 T cell-mediated cytotoxicity
Abstract
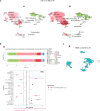
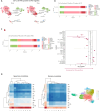
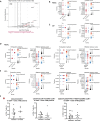
Chronic neuroinflammation and neurodegeneration are critical but unresolved drivers of disability accumulation in progressive multiple sclerosis (MS). Chronic active white matter lesions (CAL), identifiable radiologically as paramagnetic rim lesions (PRL), indicate progression-relevant chronic neuroinflammation. Using single-cell transcriptomics (scRNAseq) and T-cell receptor sequencing (scTCR-seq), we profiled cerebrospinal fluid (CSF) and blood immune cells of 34 radiologically characterized adults with MS (17 untreated, 6 treated with B-cell-depletion) and 5 healthy controls. Coupled with proteomics, we found PRL-associated enrichment of interferon (IFN) signaling and upregulation of TCR signaling in CSF and blood. This was accompanied by clonal expansion of CD8+ T effector memory (TEM) cells, with the highly expanded clonal cells exhibiting T helper type 1 (TH1) and cytotoxic profiles. Validating the cytotoxic immune profile in blood using flow cytometry, we identified a cellular correlate of PRL exhibiting features of CD8+ TEMRA cells. Despite B-cell depletion, PRL-associated neuroinflammation, driven by myeloid activation and CD8+ T-cell cytotoxicity, persisted. Serum and CSF proteomic networks showed PRL-pertinent signatures, including networks unaffected by B-cell depletion. Using in silico perturbation, we nominated therapeutic targets, including MYD88, TNF, MYC, TYK2, JAK2, and BTK, for alleviating chronic neuroinflammation in MS. Our findings highlight mechanisms of chronic neuroinflammation in MS and point to potential biomarkers for monitoring disease progression.
Conflict of interest statement
DSR has received research funding from Abata and Sanofi, related to the current study. JMD, DM, ECF were employees of Abata at the time this study was performed and may hold shares and/or stocks in the company. RMR is a venture partner at Third Rock Ventures, is a cofounder of Abata, and may hold shares and/or stocks in the company. ASB, TJT, and DO are employees of Sanofi and may hold shares and/or stocks in the company.
Figures
























References
-
- Ransohoff R. M. Multiple sclerosis: role of meningeal lymphoid aggregates in progression independent of relapse activity. Trends Immunol 44, 266–275 (2023). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
