This is a preprint.
Functional, Immunogenetic, and Structural Convergence in Influenza Immunity between Humans and Macaques
- PMID: 40568173
- PMCID: PMC12190764
- DOI: 10.1101/2025.02.21.639368
Functional, Immunogenetic, and Structural Convergence in Influenza Immunity between Humans and Macaques
Update in
-
Functional, immunogenetic, and structural convergence in influenza immunity between humans and macaques.Sci Transl Med. 2025 Sep 10;17(815):eady3570. doi: 10.1126/scitranslmed.ady3570. Epub 2025 Sep 10. Sci Transl Med. 2025. PMID: 40929243
Abstract
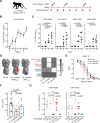
Human B cell immunity to the influenza hemagglutinin (HA) stem region, a universal influenza vaccine target, is often stereotyped and immunogenetically restricted, posing challenges for study outside humans. Here, we show that macaques vaccinated with a HA stem immunogen elicit human-like public B cell lineages targeting two major conserved sites of vulnerability, the central stem and anchor epitopes. Central stem antibodies were predominantly derived from VH1-138, the macaque homolog of human VH1-69, a VH-gene preferentially used in human central stem broadly neutralizing antibodies (bnAbs). Similarly, macaques produced anchor bnAbs with the human-like NWP motif. Both bnAb lineages were functionally and structurally analogous to their human counterparts, with recognition mediated largely by germline-encoded motifs. Thus the macaque immunoglobulin repertoire supports human-like public bnAb responses to influenza HA. Moreover, this underscores the utility of homologous germline-encoded immunity, suggesting that immune repertoires of macaques and humans may have been similarly shaped during evolution.
Keywords: B cell; Nonhuman primates; VH1–69; broadly neutralizing antibody; germline-encoded motif; hemagglutinin; influenza; public lineage; stem nanoparticle.
Conflict of interest statement
DECLARATION OF INTERESTS M.K. is named as an inventor on patents describing the HA stem immunogen used in this study under US10363301, US11147867, US11679151 which were filed by the Department of Health and Human Services.
Figures






References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
