Nile Red-Based Covalent Organic Framework as a Photocatalyst for C-H Bond Functionalization
- PMID: 40568222
- PMCID: PMC12186703
- DOI: 10.1021/acscatal.5c02173
Nile Red-Based Covalent Organic Framework as a Photocatalyst for C-H Bond Functionalization
Abstract
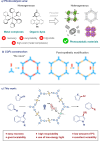
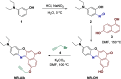
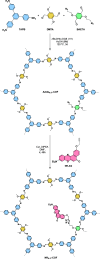
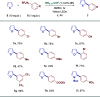
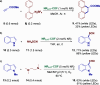
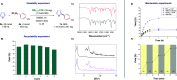
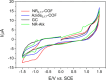
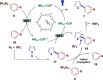
The search for efficient photocatalysts based on covalent organic frameworks (COFs) is an area of increasing interest. However, the development of these heterogeneous photocatalysts is hindered by the symmetry restrictions of the linkers used to construct these materials. Herein, we report the straightforward synthesis of an imine-based 2D-COF, NR 0.17 -COF, which incorporates a Nile Red (NR) unit via postmodification with a NR-alkyne scaffold. This framework exhibits remarkable photocatalytic activity across various photoredox-catalyzed C-H functionalization reactions, demonstrating the ability to directly functionalize prevalent bonds in organic molecules under mild conditions and with low-energy light. The NR 0.17 -COF showcases notable versatility, effectively generating aryl, sulfur, and nitrogen radicals from different radical precursors while maintaining good functional group tolerance. Moreover, our heterogeneous photocatalyst outperforms traditional homogeneous systems by addressing critical challenges such as scalability and recyclability, allowing for a 10-fold increase in the reaction scale and enabling recovery and reuse up to six times. This advancement significantly enhances the potential of COF postsynthetic modification for practical applications in organic synthesis, which marks a substantial step forward in photocatalytic technology.
Keywords: COF; C−H functionalization; Nile Red; heterogeneous catalysis; photocatalysis.
© 2025 The Authors. Published by American Chemical Society.
Figures


Similar articles
-
Engineering intermolecular C-F···C=O interactions in covalent organic framework promotes dual-path H2O2 photosynthesis for sustainable disinfection.Water Res. 2025 Jun 26;285:124112. doi: 10.1016/j.watres.2025.124112. Online ahead of print. Water Res. 2025. PMID: 40614491
-
Topology-Templated Synthesis of Dibenzo[g,p]Chrysene-Based sp2 Carbon-Linked Covalent Organic Frameworks with Kagome Lattice for Enhanced Photocatalytic Hydrogen Evolution.Adv Mater. 2025 Aug 21:e04808. doi: 10.1002/adma.202504808. Online ahead of print. Adv Mater. 2025. PMID: 40838396
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
-
Eliciting adverse effects data from participants in clinical trials.Cochrane Database Syst Rev. 2018 Jan 16;1(1):MR000039. doi: 10.1002/14651858.MR000039.pub2. Cochrane Database Syst Rev. 2018. PMID: 29372930 Free PMC article.
-
Home treatment for mental health problems: a systematic review.Health Technol Assess. 2001;5(15):1-139. doi: 10.3310/hta5150. Health Technol Assess. 2001. PMID: 11532236
References
-
- Anastas, P. T. ; Warner, J. C. . Green Chemistry: Theory and Practice; Oxford University Press: New York, 1998.
- Anastas P. T., Zimmerman J. B.. The Molecular Basis of Sustainability. Chem. 2016;1:10–12. doi: 10.1016/j.chempr.2016.06.016. - DOI
- Sheldon R. A.. Metrics of Green Chemistry and Sustainability: Past, Present, and Future. ACS Sustainable Chem. Eng. 2018;6:32–48. doi: 10.1021/acssuschemeng.7b03505. - DOI
- Zuin V. G., Eilks I., Elschami M., Kümmerer K.. Education in Green Chemistry and in Sustainable Chemistry: Perspectives towards Sustainability. Green Chem. 2021;23:1594–1608. doi: 10.1039/D0GC03313H. - DOI
-
- Shaw M. H., Twilton J., MacMillan W. C.. Photoredox Catalysis in Organic Chemistry. J. Org. Chem. 2016;81:6898–6926. doi: 10.1021/acs.joc.6b01449. - DOI - PMC - PubMed
- Marzo L., Pagire S. K., Reiser O., König B.. Visible-Light Photocatalysis: Does It Make a Difference in Organic Synthesis? Angew. Chem., Int. Ed. 2018;57:10034–10072. doi: 10.1002/anie.201709766. - DOI - PubMed
- Zhou Q.-Q., Zou Y.-Q., Lu L.-Q., Xiao W.-J.. Visible-Light-Induced Organic Photochemical Reactions through Energy-Transfer Pathways. Angew. Chem., Int. Ed. 2019;58:1586–1604. doi: 10.1002/anie.201803102. - DOI - PubMed
- McAtee R. C., McClain E. J., Stephenson C. R. J.. Illuminating Photoredox Catalysis. Trends Chem. 2019;1:111–125. doi: 10.1016/j.trechm.2019.01.008. - DOI - PMC - PubMed
- Crisenza G. E.M., Melchiorre P.. Chemistry Glows Green with Photoredox Catalysis. Nat. Commun. 2020;11:803. - PMC - PubMed
- Reischauer S., Pieber B.. Emerging Concepts in Photocatalytic Organic Synthesis. iScience. 2021;24:102209. doi: 10.1016/j.isci.2021.102209. - DOI - PMC - PubMed
- Chan A. Y., Perry I. B., Bissonnette N. B., Buksh B. F., Edwards G. A., Frye L. I., Garry O. L., Lavagnino M. N., Li B. X., Liang Y., Mao E., Millet A., Oakley J. V., Reed N. L., Sakai H. A., Seath C. P., MacMillan D. W. C.. Chem. Rev. 2022;122:1485–1542. doi: 10.1021/acs.chemrev.1c00383. - DOI - PubMed
- Dutta S., Erchinger J. E., Strieth-Kalthoff F., Kleinmans R., Glorius F.. Energy Transfer Photocatalysis: Exciting Modes of Reactivity. Chem. Soc. Rev. 2024;53:1068–1089. doi: 10.1039/D3CS00190C. - DOI - PubMed
-
- Lee J., Papatzimas J. W., Bromby A. D., Gorobets E., Derksen D. J.. Thiaporphyrin-Mediated Photocatalysis Using Red Light. RSC Adv. 2016;6:59269–59272. doi: 10.1039/C6RA11374E. - DOI
- Yerien D. E., Cooke M. V., García Vior M. C., Barata-Vallejo S., Postigo A.. Radical Fluoroalkylation Reactions of (Hetero)arenes and Sulfides under Red Light Photocatalysis. Org. Biomol. Chem. 2019;17:3741–3746. doi: 10.1039/C9OB00486F. - DOI - PubMed
- Mei L., Veleta J. M., Gianetti T. L.. Helical Carbenium Ion: A Versatile Organic Photoredox Catalyst for Red-Light-Mediated Reactions. J. Am. Chem. Soc. 2020;142:12056–12061. doi: 10.1021/jacs.0c05507. - DOI - PubMed
- Ravetz B. D., Tay N. E. S., Joe C. L., Sezen-Edmonds M., Schmidt M. A., Tan Y., Janey J. M., Eastgate M. D., Rovis T.. Development of a Platform for Near-Infrared Photoredox Catalysis. ACS Cent. Sci. 2020;6:2053–2059. doi: 10.1021/acscentsci.0c00948. - DOI - PMC - PubMed
- Glaser F., Wenger O. S.. Red Light-Based Dual Photoredox Strategy Resembling the Z-Scheme of Natural Photosynthesis. JACS Au. 2022;2:1488–1503. doi: 10.1021/jacsau.2c00265. - DOI - PMC - PubMed
-
- Photochemistry and Photophysics of Coordination Compounds I. In Topics in Current Chemistry; Balzani, V. ; Campagna, S. , Eds.; Vol. 280; Springer Berlin, 2007.
- Photochemistry and Photophysics of Coordination Compounds II. In Topics in Current Chemistry; Balzani, V. ; Campagna, S. , Eds.; Vol. 281; Springer Berlin, 2007.
- Fagnoni M., Dondi D., Ravelli D., Albini A.. Photocatalysis for the Formation of the C–C Bond. Chem. Rev. 2007;107:2725–2756. doi: 10.1021/cr068352x. - DOI - PubMed
- Prier C. K., Rankic D. A., MacMillan D. W. C.. Visible Light Photoredox Catalysis with Transition Metal Complexes: Applications in Organic Synthesis. Chem. Rev. 2013;113:5322–5366. doi: 10.1021/cr300503r. - DOI - PMC - PubMed
- Teegardin K., Day J. I., Chan J., Weaver J.. Advances in Photocatalysis: A Microreview of Visible Light Mediated Ruthenium and Iridium Catalyzed Organic Transformations. Org. Process Res. Dev. 2016;20:1156–1163. doi: 10.1021/acs.oprd.6b00101. - DOI - PMC - PubMed
- Romero N. A., Nicewicz D. A.. Organic Photoredox Catalysis. Chem. Rev. 2016;116:10075–10166. doi: 10.1021/acs.chemrev.6b00057. - DOI - PubMed
- Amos S G. E., Garreau M., Buzzetti L., Waser J.. Photocatalysis with Organic Dyes: Facile Access to Reactive Intermediates for Synthesis. Beilstein J. Org. Chem. 2020;16:1163–1187. doi: 10.3762/bjoc.16.103. - DOI - PMC - PubMed
- Vega-Peñaloza A., Mateos J., Companyó X., Escudero-Casao M., Dell’Amico L.. A Rational Approach to Organo-Photocatalysis: Novel Designs and Structure-Property Relationships. Angew. Chem., Int. Ed. 2021;60:1082–1097. doi: 10.1002/anie.202006416. - DOI - PubMed
- Filippini G., Dosso J., Prato M.. Phenols as Novel Photocatalytic Platforms for Organic Synthesis. Helv. Chim. Acta. 2023;106:e202300059
- Gentile G., Bartolomei B., Dosso J., Demitri N., Filippini G., Prato M.. Synthesis of a Novel Tetra-Phenol π-Extended Phenazine and its Application as an Organo-Photocatalyst. Chem. Commun. 2024;60:602–605. doi: 10.1039/D3CC05176E. - DOI - PubMed
LinkOut - more resources
Full Text Sources
