Intracranial volume segmentation for neurodegenerative populations using multicentre FLAIR MRI
- PMID: 40568234
- PMCID: PMC12172818
- DOI: 10.1016/j.ynirp.2021.100006
Intracranial volume segmentation for neurodegenerative populations using multicentre FLAIR MRI
Abstract
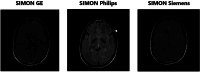
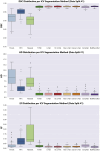
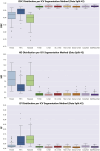
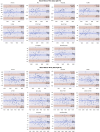
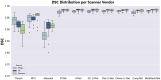
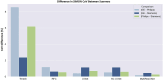
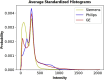
Intracranial volume (ICV) segmentation, also known as brain extraction or skull-stripping, is a critical preprocessing step in analytical pipelines for studying neurodegenerative diseases in magnetic resonance imaging (MRI). While the fluid-attenuated inversion recovery (FLAIR) MRI modality has emerged as an important sequence for analyzing cerebrovascular and neurodegenerative disease, most existing automated ICV segmentation methods have been developed for T1-weighted or multi-modal inputs. Additionally, many methods have been designed using single centre data of healthy subjects and encounter difficulties using images with varying acquisition parameters and neurodegenerative pathology. In this work, we develop and evaluate 2 traditional and 8 deep learning algorithms for ICV segmentation in FLAIR MRI. Training and testing were completed on 175 vol (8317 images) from 2 dementia and 1 vascular disease cohort. A human phantom FLAIR MRI dataset from a repeatedly scanned, healthy individual was also utilized for reliability analysis. Images were acquired from 47 imaging centres with varying scanners and parameters. To measure and compare performance, we present a novel framework for evaluating the effectiveness of computer generated segmentations on multicentre datasets. The evaluation framework includes assessments of algorithm accuracy, generalization capabilities, robustness to pathology and spatial location, and volumetric measurement reliability - all important dimensions for establishing proof of effectiveness (a prerequisite to clinical translation). The top performing method was a multiple resolution U-Net (MultiResUNet), which achieved a mean Dice similarity coefficient greater than 98% and was robust across pathology levels and spatial locations. Our results confirm a FLAIR-based ICV analytical pipeline can alone be utilized for large-scale neurodegenerative disease research. The presented evaluation framework can be deployed by other researchers to assess the viability of tools proposed for automated analysis of diverse, clinical MRI datasets.
Keywords: Brain extraction; Deep learning; FLAIR MRI; ICV segmentation; Skull-stripping.
Crown Copyright © 2021 Published by Elsevier Inc.
Conflict of interest statement
This manuscript has not been published and is not under consideration for publication elsewhere. All authors have approved the manuscript and agree with its submission to Neuroimage: Reports. We have no conflicts of interest to disclose.
Figures













Similar articles
-
Segmentation of white matter lesions in multicentre FLAIR MRI.Neuroimage Rep. 2021 Aug 5;1(4):100044. doi: 10.1016/j.ynirp.2021.100044. eCollection 2021 Dec. Neuroimage Rep. 2021. PMID: 40568442 Free PMC article.
-
Semi-Supervised Learning Allows for Improved Segmentation With Reduced Annotations of Brain Metastases Using Multicenter MRI Data.J Magn Reson Imaging. 2025 Jun;61(6):2469-2479. doi: 10.1002/jmri.29686. Epub 2025 Jan 10. J Magn Reson Imaging. 2025. PMID: 39792624 Free PMC article.
-
Cross-sectional and longitudinal Biomarker extraction and analysis for multicentre FLAIR brain MRI.Neuroimage Rep. 2022 Mar 8;2(2):100091. doi: 10.1016/j.ynirp.2022.100091. eCollection 2022 Jun. Neuroimage Rep. 2022. PMID: 40568248 Free PMC article.
-
Cost-effectiveness of using prognostic information to select women with breast cancer for adjuvant systemic therapy.Health Technol Assess. 2006 Sep;10(34):iii-iv, ix-xi, 1-204. doi: 10.3310/hta10340. Health Technol Assess. 2006. PMID: 16959170
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
References
-
- Ali H.M., Kaiser M.S., Mahmud M. International Conference on Brain Informatics. Springer; Cham: 2019. Application of convolutional neural network in segmenting brain regions from MRI data; pp. 136–146. December.
-
- Aribisala B.S., Hernández M.C.V., Royle N.A., Morris Z., Maniega S.M., Bastin M.E., et al. Brain atrophy associations with white matter lesions in the ageing brain: the Lothian Birth Cohort 1936. Eur. Radiol. 2013;23(4):1084–1092. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
