Faster decline of very prodromal dementia with Lewy bodies when amyloid positive
- PMID: 40568304
- PMCID: PMC12187975
- DOI: 10.1002/dad2.70141
Faster decline of very prodromal dementia with Lewy bodies when amyloid positive
Abstract
Introduction: The cognitive and neuroimaging evolution during dementia with Lewy bodies (DLB) from the prodromal phase (Pro-DLB; subjective cognitive impairment [SCI] to mild cognitive impairment [MCI]) according to amyloid beta (Aβ) status is poorly understood.
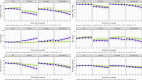
Methods: The decline of Lewy-Memento patients with SCI or MCI was compared according to Aβ status across four groups: Pro-DLB, prodromal Alzheimer's disease (Pro-AD), Pro-DLB+AD, and a group without prodromal DLB and AD (no symptoms [NS]). We observed the evolution of cognitive, functional, quality of life measures, brain volumetry, and metabolism on fluorodeoxyglucose positron emission tomography.
Results: In the Pro-DLB and Pro-DLB+AD groups, Aβ+ patients had more cognitive and functional decline than the Aβ- patients. In the Pro-AD and NS groups, Aβ+ patients had more functional decline. Aβ+ Pro-AD showed a greater volume decline of the brain (left insula).
Discussion: The presence of amyloid lesions worsens very prodromal DLB patients over time, both cognitively and functionally, but without increasing atrophy.
Highlights: Patients at a very prodromal stage, subjective cognitive impairment or mild cognitive impairment, had a clinical diagnosis of either prodromal Alzheimer's disease (Pro-AD), prodromal dementia with Lewy bodies (Pro-DLB), Pro-DLB+AD, or no diagnosis.Amyloid beta positive (Aβ+) patients had more functional decline, whatever the group.Aβ+ DLB patients (Pro-DLB and Pro-DLB+AD) had more global cognitive (Mini-Mental State Examination) decline.Aβ+ Pro-AD patients showed a greater volume decline of the left insula.
Keywords: Alzheimer's disease; amyloid; dementia with Lewy bodies; mild cognitive impairment; prodromal; subjective cognitive impairment.
© 2025 The Author(s). Alzheimer's & Dementia: Diagnosis, Assessment & Disease Monitoring published by Wiley Periodicals, LLC on behalf of Alzheimer's Association.
Conflict of interest statement
F.B. was the national coordinator for France for the Eisai Delphia (E2027), Axovant Headway‐DLB, and Roche Graduate therapeutic trials; he had received honoraria from Roche, Eisai, and Biogen for oral presentations and from Eisai for a board. The other authors declare that they have no competing interests related to the present work. Author disclosures are available in the supporting information.
Figures



References
LinkOut - more resources
Full Text Sources
Miscellaneous
