Trimethylamine-N-Oxide Impedes Late Endothelial Progenitor Cell-Mediated Revascularization by Triggering Mitochondrial Apoptosis via Suppression of MnSOD
- PMID: 40568449
- PMCID: PMC12197513
- DOI: 10.1155/cdr/9910333
Trimethylamine-N-Oxide Impedes Late Endothelial Progenitor Cell-Mediated Revascularization by Triggering Mitochondrial Apoptosis via Suppression of MnSOD
Abstract
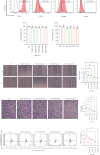
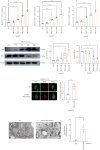
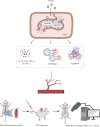
Background and Aims: Trimethylamine-N-oxide (TMAO) is recognized as a novel marker and mediator of atherosclerotic cardiovascular disease (ASCVD). Endothelial progenitor cells (EPCs) are crucial for maintaining vascular homeostasis. Impaired EPC numbers and function correlate with increased adverse cardiovascular events. The aim of this study was to decipher the effect of TMAO on late EPCs (LEPCs) and its underlying molecular mechanism. Methods and Results: In vitro migration and tubulogenic capacities of LEPCs were attenuated by TMAO in a dose-dependent manner, accompanied by inhibition of manganese superoxide dismutase (MnSOD) and mitochondrial damage. TMAO-induced mitochondrial damage provoked proinflammatory responses (increased levels of IL-6, IL-1b, ICAM-1, E-sel, and TNF-α) and autophagic cell death (confirmed by western blot immunofluorescent staining and transmission electron microscopy) in LEPCs. Overexpression of MnSOD through adenovirus transfection reversed TMAO-related LEPCs dysfunction. To study the effect of TMAO on LEPC-mediated vascular repair in vivo, a hind limb ischemia model was established in nude mice, and LEPCs were injected in the ischemic hind limb. Laser Doppler imaging of mouse ischemic hindlimbs at 21 days indicated that TMAO treatment inhibited LEPCs-mediated blood flow recovery, which was restored by MnSOD overexpression. Immunohistology analyses further revealed consistent alterations in capillary density determined by CD31 staining. Conclusions: TMAO induces mitochondrial damage in LEPCs via MnSOD suppression, which leads to cell dysfunction, proinflammatory activation, and autophagic cell death in vitro and impaired LEPCs-mediated revascularization in vivo. Overexpression of MnSOD restores TMAO-induced LEPCs dysfunction and further enhances LEPC-mediated revascularization in the ischemic hind limbs in nude mice.
Copyright © 2025 Yijia Shao et al. Cardiovascular Therapeutics published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
Rhynchophylline as an agonist of sirtuin 3 ameliorates endothelial dysfunction via antagonizing mitochondrial damage of endothelial progenitor cells.Br J Pharmacol. 2025 Aug;182(15):3476-3502. doi: 10.1111/bph.70032. Epub 2025 Mar 31. Br J Pharmacol. 2025. PMID: 40164963
-
miR-210 Regulates Autophagy Through the AMPK/mTOR Signaling Pathway, Reduces Neuronal Cell Death and Inflammatory Responses, and Enhances Functional Recovery Following Cerebral Hemorrhage in Mice.Neurochem Res. 2025 Jun 5;50(3):180. doi: 10.1007/s11064-025-04434-7. Neurochem Res. 2025. PMID: 40471451 Free PMC article.
-
DLK1 promoted ischemic angiogenesis through notch1 signaling in endothelial progenitor cells.Acta Pharmacol Sin. 2024 Dec;45(12):2553-2566. doi: 10.1038/s41401-024-01346-0. Epub 2024 Jul 26. Acta Pharmacol Sin. 2024. PMID: 39060522
-
Gut microbial metabolite trimethylamine N-oxide as a novel predictor for adverse cardiovascular events after PCI: a systematic review and dose-response meta-analysis.Nutr J. 2025 Jun 16;24(1):91. doi: 10.1186/s12937-025-01159-9. Nutr J. 2025. PMID: 40524200 Free PMC article. Review.
-
A systematic review of p53 regulation of oxidative stress in skeletal muscle.Redox Rep. 2018 Dec;23(1):100-117. doi: 10.1080/13510002.2017.1416773. Epub 2018 Jan 3. Redox Rep. 2018. PMID: 29298131 Free PMC article.
References
-
- Senthong V., Wang Z., Li X. S., et al. Intestinal Microbiota-Generated Metabolite Trimethylamine-N-Oxide and 5-Year Mortality Risk in Stable Coronary Artery Disease: The Contributory Role of Intestinal Microbiota in a COURAGE-Like Patient Cohort. Journal of the American Heart Association . 2016;5(6) doi: 10.1161/JAHA.115.002816. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

