Lactobacillus delbrueckii subsp. bulgaricus 2038 and Streptococcus thermophilus 1131 ameliorate barrier dysfunction in human induced pluripotent stem cell-derived crypt-villus structural small intestine
- PMID: 40568574
- PMCID: PMC12190435
- DOI: 10.3389/fimmu.2025.1585007
Lactobacillus delbrueckii subsp. bulgaricus 2038 and Streptococcus thermophilus 1131 ameliorate barrier dysfunction in human induced pluripotent stem cell-derived crypt-villus structural small intestine
Abstract
Background: Lactic acid bacteria (LAB) have been widely used as probiotics which contribute to our health. We previously reported that Lactobacillus delbrueckii subsp. bulgaricus 2038 and Streptococcus thermophilus 1131, two yogurt starter strains, ameliorate the intestinal barrier dysfunction caused by tumor necrosis factor (TNF)-α and interferon (IFN)-γ in Caco-2 cells. However, Caco-2 cells differ from living organisms in various ways. We have developed a human induced pluripotent stem cell-derived crypt-villus structural small intestine (hiPSC-SI) was established with a villus-like structure containing constituent cells of the small intestine.
Methods: A hiPSC-SI and LAB co-culture model was established to assess the impact of LAB on barrier function and elucidate the underlying mechanisms.
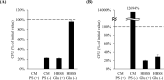
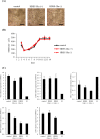
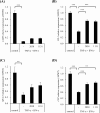
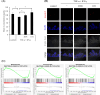
Results: The medium on the luminal side for co-culturing cells and bacteria was examined and determined to use Hanks' balanced salt solution without glucose in terms of bacterial survival rate. LAB were found to ameliorate permeability and decrease the gene expression of tight junction associated proteins induced by TNF-α and IFN-γ. Regarding cell differentiation, LAB suppressed the downregulation of LGR5, VIL1, LYZ and MUC2 by cytokines. Moreover, they ameliorated reduced mucin 2 protein production and decreased the number of mucin 2-positive cells. Finally, transcriptome analysis suggested that they ameliorated the aberration in cytokine-induced cell differentiation via an anti-inflammatory effect on intestinal stem cells.
Conclusions: The results indicate that LAB ameliorate the cytokine-induced dysfunction of intestinal barrier integrity and homeostasis disrupted by cytokines in a co-culture model of hiPSC-SI and LAB.
Keywords: anti-inflammation; barrier function; differentiation; induced pluripotent stem cell-derived crypt-villus structural small intestine; lactic acid bacteria; mucin 2; tight junction.
Copyright © 2025 Kobayashi, Imai, Shimizu, Ogawa, Nakai, Mizuno, Kaneda, Watanabe-Yasuoka, Yamazaki, Mochizuki, Sashihara, Matsunaga and Iwao.
Conflict of interest statement
KK, YW-Y, FY, and TS are employees of Meiji Holdings Co., Ltd. JM is employee of Meiji Co. Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Yogurt starter strains ameliorate intestinal barrier dysfunction via activating AMPK in Caco-2 cells.Tissue Barriers. 2024 Jan 2;12(1):2184157. doi: 10.1080/21688370.2023.2184157. Epub 2023 Feb 28. Tissue Barriers. 2024. PMID: 36852963 Free PMC article.
-
Lactobacillus delbrueckii subsp. bulgaricus 2038 and Streptococcus thermophilus 1131 Induce the Expression of the REG3 Family in the Small Intestine of Mice via the Stimulation of Dendritic Cells and Type 3 Innate Lymphoid Cells.Nutrients. 2019 Dec 7;11(12):2998. doi: 10.3390/nu11122998. Nutrients. 2019. PMID: 31817820 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
-
Lactiplantibacillus plantarum strengthens the intestinal barrier: involvement of the endocannabinoidome.Am J Physiol Gastrointest Liver Physiol. 2025 Aug 1;329(2):G245-G260. doi: 10.1152/ajpgi.00142.2024. Epub 2025 Jun 16. Am J Physiol Gastrointest Liver Physiol. 2025. PMID: 40522902
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
References
-
- Kobayashi K, Honme Y, Sashihara T. Lactobacillus delbrueckii subsp. bulgaricus 2038 and Streptococcus thermophilus 1131 induce the expression of the REG3 family in the small intestine of mice via the stimulation of dendritic cells and type 3 innate lymphoid cells. Nutrients. (2019) 11:2998. doi: 10.3390/nu11122998 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

