Unraveling the immune mechanisms and therapeutic targets in lung adenosquamous transformation
- PMID: 40568576
- PMCID: PMC12188374
- DOI: 10.3389/fimmu.2025.1542526
Unraveling the immune mechanisms and therapeutic targets in lung adenosquamous transformation
Abstract
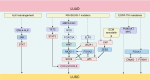
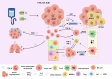
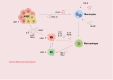
Adenocarcinoma-to-squamous cell carcinoma transformation (AST) induces drug resistance in patients with lung adenocarcinoma (LUAD), often resulting in unfavorable clinical outcomes. In recent years, it has been found that alterations in the tumor immune microenvironment (TIME) during adenosquamous carcinoma trans-differentiation also influence the efficacy of immunotherapy. Moreover, the aberrant expression and activation of several driver genes for AST lead to abnormal infiltration and function of immune cell by remodeling the cellular inflammatory phenotype. In this review, we will systematically present the changes in the TIME and molecular regulatory mechanisms during adenosquamous carcinoma differentiation, aiming to gain a better understand of the function of immune cells during this process and the potential value of combining immunotherapy to enhance the treatment of non-small cell lung cancer (NSCLC).
Keywords: adenocarcinoma-to-squamous cell carcinoma transformation (AST); adenosquamous lung carcinoma (ASLC); tumor immune microenvironment (TIME); tumor-associated macrophages (TAMs); tumor-associated neutrophils (TANs).
Copyright © 2025 Xu, Yang, Wang, Lin, Zhang, Ni and Xu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Attenuated immune surveillance during squamous cell transformation of pancreatic adenosquamous cancer defines new therapeutic opportunity for cancer interception.J Immunother Cancer. 2025 Jun 23;13(6):e012066. doi: 10.1136/jitc-2025-012066. J Immunother Cancer. 2025. PMID: 40550564 Free PMC article.
-
Unraveling the role of GPCR signaling in metabolic reprogramming and immune microenvironment of lung adenocarcinoma: a multi-omics study with experimental validation.Front Immunol. 2025 Jun 6;16:1606125. doi: 10.3389/fimmu.2025.1606125. eCollection 2025. Front Immunol. 2025. PMID: 40547013 Free PMC article.
-
Applications of single-cell analysis in immunotherapy for lung cancer: Current progress, new challenges and expectations.J Adv Res. 2025 Aug;74:269-281. doi: 10.1016/j.jare.2024.10.008. Epub 2024 Oct 12. J Adv Res. 2025. PMID: 39401694 Free PMC article. Review.
-
Immunotherapy (excluding checkpoint inhibitors) for stage I to III non-small cell lung cancer treated with surgery or radiotherapy with curative intent.Cochrane Database Syst Rev. 2017 Dec 16;12(12):CD011300. doi: 10.1002/14651858.CD011300.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2021 Dec 6;12:CD011300. doi: 10.1002/14651858.CD011300.pub3. PMID: 29247502 Free PMC article. Updated.
-
Development of a machine learning-derived dendritic cell signature for prognostic stratification in lung adenocarcinoma.Front Immunol. 2025 Jun 9;16:1621370. doi: 10.3389/fimmu.2025.1621370. eCollection 2025. Front Immunol. 2025. PMID: 40552290 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

