This is a preprint.
Inborn errors of immunity: manifestation, treatment, and outcome - an ESID registry 1994-2024 report on 30,628 patients
- PMID: 40568655
- PMCID: PMC12191083
- DOI: 10.1101/2025.02.20.25322586
Inborn errors of immunity: manifestation, treatment, and outcome - an ESID registry 1994-2024 report on 30,628 patients
Abstract
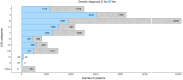
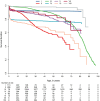
The European Society for Immunodeficiencies patient registry (ESID-R), established in 1994, is one of the world's largest databases on inborn errors of immunity (IEI). IEI are genetic disorders predisposing patients to infections, autoimmunity, inflammation, allergies and malignancies. Treatments include antimicrobial therapy, immunoglobulin replacement, immune modulation, stem cell transplantation and gene therapy. Data from 194 centers in 33 countries capture clinical manifestations and treatments from birth onward, with annually expected updates. This report reviews the ESID-R's structure, data content, and impact. The registry includes 30,628 patient datasets (aged 0-97.9 years; median follow-up: 7.2 years; total 825,568.2 patient-years), with 13,550 cases in 15 sub-studies. It has produced 84 peer-reviewed publications (mean citation rate: 95). Findings include real-world observations of IEI diagnoses, genetic causes, clinical manifestations, treatments, and survival trends. The ESID-R fosters global collaboration, advancing IEI research and patient care. This report highlights the key role of the multi-national ESID-R, led by an independent medical society, in evidence-based discovery.
Keywords: Inborn error of immunity (IEI); online database; overall survival; patient registry; primary immune disorder (PID); primary immunodeficiency (PID).
Conflict of interest statement
MGS received advisory board honoraria from Pharming. MHA received research support, consulting fees, and speaker honoraria from Octapharma. GK, FC declare no conflict of interest. LRD KU Leuven receives research funding and advisory board honoraria for IM from CSL-Behring and Boehringer-Ingelheim. BS has received travel grants from Pharming, and speaker honoraria from Takeda.Conflict of interest statement at the end of the article.
Figures





References
-
- Hagele P, Staus P, Scheible R, Uhlmann A, Heeg M, Klemann C, Maccari ME, Ritterbusch H, Armstrong M, Cutcutache I, Elliott KS, von Bernuth H, Leahy TR, Leyh J, Holzinger D, Lehmberg K, Svec P, Masjosthusmann K, Hambleton S, Jakob M, Sparber-Sauer M, Kager L, Puzik A, Wolkewitz M, Lorenz MR, Schwarz K, Speckmann C, Rensing-Ehl A, Ehl S, group As. Diagnostic evaluation of paediatric autoimmune lymphoproliferative immunodeficiencies (ALPID): a prospective cohort study. Lancet Haematol 2024;11(2):e114–e26 doi: 10.1016/S2352-3026(23)00362-9. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
