AliC and AliD of nonencapsulated Streptococcus pneumoniae enhance virulence in a Galleria mellonella model of infection by contributing to reactive oxygen species resistance
- PMID: 40568701
- PMCID: PMC12187840
- DOI: 10.3389/fcimb.2025.1583375
AliC and AliD of nonencapsulated Streptococcus pneumoniae enhance virulence in a Galleria mellonella model of infection by contributing to reactive oxygen species resistance
Abstract
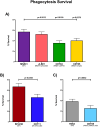
Introduction: Nonencapsulated Streptococcus pneumoniae (NESp) are isolated worldwide. Due to the lack of capsule in NESp strains the current vaccines, that target the pneumococcal capsule are ineffective. Some NESp contain the oligopeptide transporters AliC and AliD which are required for virulence through unknown mechanisms. AliC and AliD have been previously shown to reduce rates of phagocytosis and alter the transcriptome and proteome of MNZ41. We hypothesize that oligopeptide regulated genes are responsible for reduced phagocytosis and increased survival through resistance to reactive oxygen species (ROS).
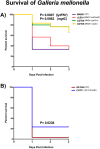
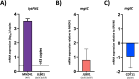
Methods: To test this a mutant library of AliC and AliD regulated genes was used in in vitro and in vivo models. ROS resistance was tested through quantifying bacterial counts after exposure to hydrogen peroxide (H2O2). A modified surface killing assay was also used to calculate resistance to phagocytosis of our mutant library. A Galleria mellonella larvae model of infection was used to determine survival curve analyses.
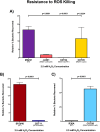
Results: Two mutant genes in our library, ∆lytFN1 (CDT04) and ∆mgtC (CDT05), displayed greater sensitivity to H2O2 killing and phagocytosis compared to wildtype MNZ41. Deletion of AliD in an AliD-expressing encapsulated strain reduced virulence.
Conclusion: This research demonstrates that proteins encoded by genes regulated by AliC and AliD alter susceptibility to host-derived mechanisms for bacterial clearance and increases bacterial survival in response to ROS.
Keywords: Galleria mellonella; Streptococcus pneumoniae; host-pathogen; oligopeptide transporter; virulence factor.
Copyright © 2025 Thompson, Khan, Crosby, Holcomb, Vidal, Vidal, McDaniel and Keller.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures


Similar articles
-
Mucosal Infections and Invasive Potential of Nonencapsulated Streptococcus pneumoniae Are Enhanced by Oligopeptide Binding Proteins AliC and AliD.mBio. 2018 Jan 16;9(1):e02097-17. doi: 10.1128/mBio.02097-17. mBio. 2018. PMID: 29339428 Free PMC article.
-
An abundance of aliC and aliD genes were identified in saliva using a novel multiplex qPCR to characterize group II non-encapsulated pneumococci with improved specificity.Microbiology (Reading). 2025 Apr;171(4):001555. doi: 10.1099/mic.0.001555. Microbiology (Reading). 2025. PMID: 40279151 Free PMC article.
-
Oligopeptide Transporters of Nonencapsulated Streptococcus pneumoniae Regulate CbpAC and PspA Expression and Reduce Complement-Mediated Clearance.mBio. 2023 Feb 28;14(1):e0332522. doi: 10.1128/mbio.03325-22. Epub 2023 Jan 10. mBio. 2023. PMID: 36625598 Free PMC article.
-
Immunogenicity and seroefficacy of pneumococcal conjugate vaccines: a systematic review and network meta-analysis.Health Technol Assess. 2024 Jul;28(34):1-109. doi: 10.3310/YWHA3079. Health Technol Assess. 2024. PMID: 39046101 Free PMC article.
-
Cost-effectiveness of using prognostic information to select women with breast cancer for adjuvant systemic therapy.Health Technol Assess. 2006 Sep;10(34):iii-iv, ix-xi, 1-204. doi: 10.3310/hta10340. Health Technol Assess. 2006. PMID: 16959170
References
-
- Avery O. T., MacLeod C. M., McCarty M. (1944). Studies on the chemical nature of the substance inducing transformation of pneumococcal types: Induction of transformation by a desoxyribonucleic acid fraction isolated from pneumococcus Type III. J. Exp. Med. 79, 137–158. doi: 10.1084/jem.79.2.137 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

