Germline and somatic genetic landscape of pediatric myelodysplastic syndromes
- PMID: 40568716
- PMCID: PMC12399944
- DOI: 10.3324/haematol.2024.285700
Germline and somatic genetic landscape of pediatric myelodysplastic syndromes
Abstract
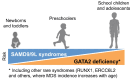
Pediatric myelodysplastic syndromes (MDS) represent a rare group of clonal hematopoietic stem cell disorders accounting for approximately 5% of pediatric hematologic malignancies. They are characterized by ineffective hematopoiesis, cytopenia, and dysplastic changes in the bone marrow with variable risk of progression to acute myeloid leukemia. Unlike adult MDS, pediatric cases predominantly present with hypocellular bone marrow, with monosomy 7 and trisomy 8 as the most common cytogenetic aberrations. Pediatric MDS can manifest as primary disease or arise secondary to classical inherited bone marrow failure syndromes, prior cytotoxic therapy, or acquired aplastic anemia. In recent years, new germline syndromes have been identified in a substantial proportion of patients with "primary" MDS. The most common are GATA2 deficiency and SAMD9/SAMD9L syndromes, accounting for at least 7% and 8% of cases, respectively. The somatic mutational landscape is different from adult MDS, with recurrent mutations affecting SETBP1, ASXL1, RUNX1, and RAS pathway genes (PTPN11, NRAS, KRAS, CBL), while mutations in spliceosome components and epigenetic regulators, which are common in adults, are virtually absent in children. Monosomy 7 serves as a "central hub" in disease evolution, associating with somatic leukemia driver mutations. On the other hand, somatic UBTF-TD and NPM1 mutations define a subtype of MDS with excess blasts with predominantly normal karyotype without known germline predisposition. Hematopoietic stem cell transplantation is the only curative option for pediatric MDS. Understanding the unique genetic profile of pediatric MDS has implications for diagnosis, therapy, donor selection and long-term surveillance, particularly for patients with germline predisposition syndromes. This review discusses current classification systems (WHO and ICC), provides a detailed overview of the germline and somatic genetic landscape of pediatric MDS, and highlights clinical implications of these genetic alterations.
Figures




Similar articles
-
Update on Recommendations for Surveillance for Children with Predisposition to Hematopoietic Malignancy.Clin Cancer Res. 2024 Oct 1;30(19):4286-4295. doi: 10.1158/1078-0432.CCR-24-0685. Clin Cancer Res. 2024. PMID: 39078402 Free PMC article. Review.
-
DBA Syndrome.2009 Jun 25 [updated 2025 Jul 31]. In: Adam MP, Feldman J, Mirzaa GM, Pagon RA, Wallace SE, Amemiya A, editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993–2025. 2009 Jun 25 [updated 2025 Jul 31]. In: Adam MP, Feldman J, Mirzaa GM, Pagon RA, Wallace SE, Amemiya A, editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993–2025. PMID: 20301769 Free Books & Documents. Review.
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Allogeneic Hematopoietic Cell Transplant For Bone Marrow Failure or Myelodysplastic Syndrome in Dyskeratosis Congenita/Telomere Biology Disorders: Single-Center, Single-Arm, Open-Label Trial of Reduced-Intensity Conditioning Without Radiation.Transplant Cell Ther. 2024 Oct;30(10):1005.e1-1005.e17. doi: 10.1016/j.jtct.2024.07.007. Epub 2024 Jul 11. Transplant Cell Ther. 2024. PMID: 39002862 Clinical Trial.
-
Long-Term Transfusion Independence with Luspatercept Versus Epoetin Alfa in Erythropoiesis-Stimulating Agent-Naive, Lower-Risk Myelodysplastic Syndromes in the COMMANDS Trial.Adv Ther. 2025 Jul;42(7):3576-3589. doi: 10.1007/s12325-025-03208-5. Epub 2025 May 16. Adv Ther. 2025. PMID: 40377899 Free PMC article. Clinical Trial.
References
-
- Hasle H, Kerndrup G, Jacobsen BB. Childhood myelodysplastic syndrome in Denmark: incidence and predisposing conditions. Leukemia. 1995;9(9):1569-1572. - PubMed
-
- Hasle H, Wadsworth LD, Massing BG, McBride M, Schultz KR. A population-based study of childhood myelodysplastic syndrome in British Columbia, Canada. Br J Haematol. 1999;106(4):1027-1032. - PubMed
-
- Sasaki H, Manabe A, Kojima S, et al. Myelodysplastic syndrome in childhood: a retrospective study of 189 patients in Japan. Leukemia. 2001;15(11):1713-1720. - PubMed
-
- Xavier AC, Kutny M, Costa LJ. Incidence and outcomes of paediatric myelodysplastic syndrome in the United States. Br J Haematol. 2018;180(6):898-901. - PubMed
-
- Niemeyer CM, Baumann I. Myelodysplastic syndrome in children and adolescents. Semin Hematol. 2008;45(1):60-70. - PubMed
Publication types
MeSH terms
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

