Leukotrienes: Bridging the Inflammatory Gap in Asthma and Inflammatory Bowel Diseases (IBD)
- PMID: 40568744
- PMCID: PMC12199122
- DOI: 10.1002/cph4.70022
Leukotrienes: Bridging the Inflammatory Gap in Asthma and Inflammatory Bowel Diseases (IBD)
Abstract
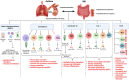
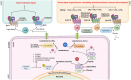
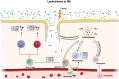
Leukotrienes are potent inflammatory lipid mediators produced primarily by immune cells. Inflammation, being the center stone of two major disease conditions, namely, asthma and inflammatory bowel disease (IBD), has led researchers to study the role of leukotrienes (LTs) in both these disease settings extensively. Several studies indicate a crucial role for LTs in the development and progression of IBD, whereas LTs, especially cysteinyl leukotrienes (cys-LTs), have been identified as the major contributors to asthma initiation and progression for over three decades. Additionally, the lungs and the gut share several common characteristics, including their exposure to the external environment, similar microbiome composition, and inflammatory responses. These similarities suggest a bidirectional relationship, supported by the increased risk of IBD in asthma patients and vice versa. However, the specific role of LTs in this lung-gut connection remains unclear. This review will examine how several common factors, such as physiology, microbiome, environment, and inflammatory mediators, especially LTs, modulate this crosstalk. The review also highlights in detail how altered leukotriene biosynthesis and signaling contribute to the pathogenesis of both asthma and IBD. Furthermore, we will consider the therapeutic implications of targeting leukotriene pathways for patients with concurrent asthma and IBD in the hope of developing more efficient treatment outcomes for these interconnected conditions. Finally, this review will very briefly explore the involvement of neuronal connections in mediating the lung-gut crosstalk.
Keywords: asthma; chronic inflammation; inflammatory bowel disease; inter‐organ communication; leukotrienes; lung–gut axis.
© 2025 The Author(s). Comprehensive Physiology published by Wiley Periodicals LLC on behalf of American Physiological Society.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Unraveling cysteinyl leukotrienes and their receptors in inflammation through the brain-gut-lung axis.Virulence. 2025 Dec;16(1):2502555. doi: 10.1080/21505594.2025.2502555. Epub 2025 May 12. Virulence. 2025. PMID: 40351036 Free PMC article. Review.
-
Addition of anti-leukotriene agents to inhaled corticosteroids for adults and adolescents with persistent asthma.Cochrane Database Syst Rev. 2017 Mar 16;3(3):CD010347. doi: 10.1002/14651858.CD010347.pub2. Cochrane Database Syst Rev. 2017. PMID: 28301050 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Microbiota-derived metabolites in inflammatory bowel disease.Semin Immunopathol. 2025 Mar 4;47(1):19. doi: 10.1007/s00281-025-01046-9. Semin Immunopathol. 2025. PMID: 40032666 Free PMC article. Review.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

