Top-Down Proteomics for the Characterization and Quantification of Calreticulin Arginylation
- PMID: 40568806
- PMCID: PMC12329857
- DOI: 10.1021/acs.analchem.4c04141
Top-Down Proteomics for the Characterization and Quantification of Calreticulin Arginylation
Abstract
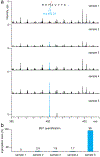
Arginylation installed by arginyltransferase 1 (ATE1) features an addition of arginine (Arg) to the reactive amino acids (e.g., Glu and Asp) at the protein N-terminus or side chain. Systemic removal of arginylation after ATE1 knockout (KO) in mouse models resulted in heart defects leading to embryonic lethality. The biological importance of arginylation has motivated the discovery of arginylation sites on proteins using bottom-up approaches. While bottom-up proteomics is powerful in localizing peptide arginylation, it lacks the ability to quantify proteoforms at the protein level. Here we developed a top-down proteomics workflow for characterizing and quantifying calreticulin (CALR) arginylation. To generate fully arginylated CALR (R-CALR), we have inserted an R residue after the signaling peptide (AA1-17). Upon overexpression in ATE1 KO cells, CALR and R-CALR were purified by affinity purification and analyzed by LCMS in positive mode. Both proteoforms showed charge states ranging from 27 to 68 with charge 58 as the most intense charge state. Their MS2 spectra from electron-activated dissociation (EAD) showed preferential fragmentation at the protein N-terminals which yielded sufficient c ions facilitating precise localization of the arginylation sites. The calcium-binding domain (CBD) gave minimum characteristic ions possibly due to the abundant presence of >100 D and E residues. Ultraviolet photodissociation (UVPD) compared with EAD and ETD significantly improved the sequence coverage of CBD. This method can identify and quantify CALR arginylation at absence, endogenous (low), and high levels. To our knowledge, our work is the first application of top-down proteomics in characterizing post-translational arginylation in vitro and in vivo.
Figures





Update of
-
Top-down Proteomics for the Characterization and Quantification of Calreticulin Arginylation.bioRxiv [Preprint]. 2024 Aug 8:2024.08.08.607245. doi: 10.1101/2024.08.08.607245. bioRxiv. 2024. Update in: Anal Chem. 2025 Jul 29;97(29):15562-15569. doi: 10.1021/acs.analchem.4c04141. PMID: 39149376 Free PMC article. Updated. Preprint.
References
-
- Yoo YD; Mun SR; Ji CH; Sung KW; Kang KY; Heo AJ; Lee SH; An JY; Hwang J; Xie XQ; Ciechanover A; Kim BY; Kwon YT N-terminal arginylation generates a bimodal degron that modulates autophagic proteolysis. Proc Natl Acad Sci U S A 2018, 115 (12), E2716–E2724. DOI: 10.1073/pnas.1719110115 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

