RIG-I-Inducing Small Molecules Potently Inhibit HMA-Resistant AML Through Igniting the Overloaded dsRNA Arsenal
- PMID: 40568903
- PMCID: PMC12463013
- DOI: 10.1002/advs.202414477
RIG-I-Inducing Small Molecules Potently Inhibit HMA-Resistant AML Through Igniting the Overloaded dsRNA Arsenal
Abstract
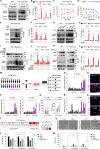
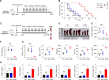
DNA hypomethylating agents (HMAs) are widely used to treat acute myeloid leukemia (AML)/myelodysplastic syndrome (MDS), but most treated patients relapse and lack standard treatment options. Using high-throughput screening, the approved all-trans retinoic acid (ATRA) is identified that exhibit high selectivity in killing HMA-resistant AML cells compared to parental cells. Mechanistically, HMA-resistant cells are overloaded with DNA hypomethylation-associated endogenous viral double-stranded RNA (dsRNA) which, however, fails to trigger an anticancer interferon (IFN) immune response due to downregulation of dsRNA sensor retinoic acid-inducible gene I (RIG-I). ATRA compensates for RIG-I expression, thereby re-triggering IFN response and potently inhibiting HMA-resistant AML cell lines, xenograft mice, and patient-derived primary cells. A library of potential RIG-I-inducing compounds is rationally constructed and screened, in which the approved M3 AML treatment drug tamibarotene (TAM) exhibits strikingly 28036-fold selectivity and 779 pm IC50 in killing HMA-resistant AML cells. ATRA and TAM do not selectively inhibit p53-mutant cancer cells. Together, this study uncovers a common resistance mechanism in HMA-treated AML patients and, in addition, provides highly potent and selective agents that can overcome resistance through re-triggering IFN anticancer immune response.
Keywords: RIG‐I; acute myeloid leukemia; anticancer immune response; hypomethylating agent; interferon.
© 2025 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Chiappinelli K. B., Strissel P. L., Desrichard A., Li H., Henke C., Akman B., Hein A., Rote N. S., Cope L. M., Snyder A., Makarov V., Budhu S., Slamon D. J., Wolchok J. D., Pardoll D. M., Beckmann M. W., Zahnow C. A., Merghoub T., Chan T. A., Baylin S. B., Strick R., Cell 2015, 162, 974. - PMC - PubMed
-
- Welch J. S., Petti A. A., Miller C. A., Fronick C. C., O'Laughlin M., Fulton R. S., Wilson R. K., Baty J. D., Duncavage E. J., Tandon B., Lee Y. S., Wartman L. D., Uy G. L., Ghobadi A., Tomasson M. H., Pusic I., Romee R., Fehniger T. A., Stockerl‐Goldstein K. E., Vij R., Oh S. T., Abboud C. N., Cashen A. F., Schroeder M. A., Jacoby M. A., Heath S. E., Luber K., Janke M. R., Hantel A., Khan N., et al., The New England J. Med. 2016, 375, 2023. - PMC - PubMed
MeSH terms
Substances
Grants and funding
- 82425001/National Natural Science Foundation of China
- 82130075/National Natural Science Foundation of China
- 823B2004/National Natural Science Foundation of China
- 82472578/National Natural Science Foundation of China
- 2023ZD0501300/Noncommunicable Chronic Diseases-National Science and Technology Major Project
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
