External validation of QUiPP App in three independent European cohorts of symptomatic women
- PMID: 40568915
- PMCID: PMC12317302
- DOI: 10.1002/uog.29263
External validation of QUiPP App in three independent European cohorts of symptomatic women
Abstract
Objective: To validate externally the QUantitative Innovation in Predicting Preterm birth (QUiPP) App v.2 for the prediction of spontaneous preterm birth (sPTB) in symptomatic women attending tertiary care in Europe.
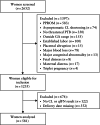
Methods: The QUiPP App v.2 was validated in three independent datasets: a prospective European multicenter cohort across five countries (n = 452), a retrospective single-center cohort in The Netherlands (n = 581) and a retrospective single-center cohort in Belgium (n = 399). The cohorts consisted of pregnant women between 23 and 34 weeks' gestation with symptoms of threatened preterm birth attending a tertiary care hospital between 2012 and 2023. We calculated risk estimates using the QUiPP App v.2 by inputting quantitative fetal fibronectin (qfFN) and/or cervical length (CL) measurement, in addition to other risk factors. As a result of the absence of a fibronectin detection kit in the Belgian cohort, only the QUiPP model based on CL could be validated in this dataset. The European cohort had no missing cases, but for the Dutch cohort, only complete cases were analyzed due to missing data. For the Belgian cohort, we statistically corrected for patients lost to follow-up using inverse probability of censoring weighting. Discrimination was assessed using receiver-operating-characteristics (ROC)-curve analysis of three QUiPP models (qfFN alone, CL alone and CL plus qfFN) for the risk of sPTB at six predefined timepoints: within 1, 2 and 4 weeks after testing, and at < 30, < 34 and < 37 weeks' gestation. Sensitivity, specificity and positive and negative likelihood ratios were calculated using risk thresholds of 5%, 10% and 15%. Model calibration was assessed to evaluate the agreement between expected and observed outcomes.
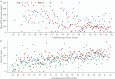
Results: The predictive performance of the QUiPP App v.2 for sPTB within 1 week after testing had an area under the ROC curve (AUC) of 0.84 (95% CI, 0.79-0.89) and 0.74 (95% CI, 0.66-0.83) in the European and Dutch cohorts, respectively, using the combined model of CL plus qfFN, and 0.80 (95% CI, 0.75-0.85) in the Belgian cohort using the CL-only model. Predictive performance was greater for shorter-term outcomes, specifically sPTB < 30 weeks, compared with longer-term outcomes, such as sPTB < 37 weeks. The highest AUC (0.91 (95% CI, 0.86-0.95)) was achieved by the model using CL plus qfFN for the prediction of sPTB < 30 weeks in the European cohort. Calibration was excellent for patients with negligible risk; however, for women at greater risk of sPTB, the risk was generally underestimated compared with the observed event rate.
Conclusions: The QUiPP App v.2 offers reassurance for patients with low predicted risk of sPTB and has greater predictive performance for shorter-term, compared with longer-term, outcomes. Despite significant differences in prevalence from the original QUiPP dataset, the model combining CL plus qfFN and the qfFN-only model perform reasonably well. Statistical correction for patients lost to follow-up in the dataset comprising censored and uncensored patients improved the discriminative ability of the CL predictor. © 2025 The Author(s). Ultrasound in Obstetrics & Gynecology published by John Wiley & Sons Ltd on behalf of International Society of Ultrasound in Obstetrics and Gynecology.
Keywords: prediction; risk assessment; spontaneous preterm birth; validation.
© 2025 The Author(s). Ultrasound in Obstetrics & Gynecology published by John Wiley & Sons Ltd on behalf of International Society of Ultrasound in Obstetrics and Gynecology.
Conflict of interest statement
A.M.F., A.L.R. and P.C.A.M.B. are funded by a grant of public–private partnerships (PPP) from Amsterdam University Medical Center (identifier 2007651). The collaboration project is cofunded by the PPP Allowance made available by Health~Holland, Top Sector Life Sciences & Health. This study was performed in the context of the COCOON (combining cord‐free uterine electrohysterography and standard clinical measurements for refining the detection of premature birth) Study, a cooperation of Stichting VUmc, Stichting VU, Health~Holland and Bloom Technologies NV. These funding bodies played no role in the preparation of this paper. B.W.M. reports consultancy, travel support and research funding from Merck and consultancy for Organon and Norgine. B.W.M. holds stock from ObsEva and is supported by a NHMRC Practitioner Fellowship (GNT1082548).
A.M.F., A.L.R. and P.C.A.M.B. are funded by a grant of public–private partnerships (PPP) from Amsterdam University Medical Center (identifier 2007651). The collaboration project is cofunded by the PPP Allowance made available by Health~Holland, Top Sector Life Sciences & Health. This study was performed in the context of the COCOON (combining cord‐free uterine electrohysterography and standard clinical measurements for refining the detection of premature birth) Study, a cooperation of Stichting VUmc, Stichting VU, Health~Holland and Bloom Technologies NV. These funding bodies played no role in the preparation of this paper. B.W.M. reports consultancy, travel support and research funding from Merck and consultancy for Organon and Norgine. B.W.M. holds stock from ObsEva and is supported by a NHMRC Practitioner Fellowship (GNT1082548).
Figures
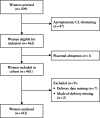
 ), Amsterdam University Medical Center dataset (
), Amsterdam University Medical Center dataset ( ) and Ghent University Hospital dataset (
) and Ghent University Hospital dataset ( ). qfFN was not analyzed in the Ghent University Hospital dataset.
). qfFN was not analyzed in the Ghent University Hospital dataset.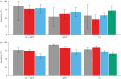
 ), European Fibronectin Study dataset (
), European Fibronectin Study dataset ( ), Amsterdam University Medical Center dataset (
), Amsterdam University Medical Center dataset ( ) and Ghent University Hospital dataset (
) and Ghent University Hospital dataset ( ).
).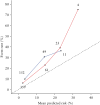
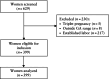
 ) and Amsterdam University Medical Center dataset (
) and Amsterdam University Medical Center dataset ( ). Numbers of samples are annotated. Dashed line indicates perfect calibration.
). Numbers of samples are annotated. Dashed line indicates perfect calibration.Similar articles
-
Development and validation of predictive models for QUiPP App v.2: tool for predicting preterm birth in asymptomatic high-risk women.Ultrasound Obstet Gynecol. 2020 Mar;55(3):348-356. doi: 10.1002/uog.20401. Ultrasound Obstet Gynecol. 2020. PMID: 31325332
-
Predictive value of cervical length for spontaneous preterm birth in women with cervical cerclage.Ultrasound Obstet Gynecol. 2025 Aug;66(2):210-216. doi: 10.1002/uog.29281. Epub 2025 Jul 9. Ultrasound Obstet Gynecol. 2025. PMID: 40631398 Free PMC article.
-
Development and validation of predictive models for QUiPP App v.2: tool for predicting preterm birth in women with symptoms of threatened preterm labor.Ultrasound Obstet Gynecol. 2020 Mar;55(3):357-367. doi: 10.1002/uog.20422. Ultrasound Obstet Gynecol. 2020. PMID: 31385343
-
Cervical length screening for prevention of preterm birth in singleton pregnancy with threatened preterm labor: systematic review and meta-analysis of randomized controlled trials using individual patient-level data.Ultrasound Obstet Gynecol. 2017 Mar;49(3):322-329. doi: 10.1002/uog.17388. Epub 2017 Feb 8. Ultrasound Obstet Gynecol. 2017. PMID: 27997053
-
Planned early birth versus expectant management for women with preterm prelabour rupture of membranes prior to 37 weeks' gestation for improving pregnancy outcome.Cochrane Database Syst Rev. 2017 Mar 3;3(3):CD004735. doi: 10.1002/14651858.CD004735.pub4. Cochrane Database Syst Rev. 2017. PMID: 28257562 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

