Rapamycin Alleviates Heart Failure Caused by Mitochondrial Dysfunction and SERCA Hypoactivity in Syntaxin 12/13 Deficient Models
- PMID: 40568929
- PMCID: PMC12376582
- DOI: 10.1002/advs.202507210
Rapamycin Alleviates Heart Failure Caused by Mitochondrial Dysfunction and SERCA Hypoactivity in Syntaxin 12/13 Deficient Models
Abstract
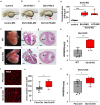
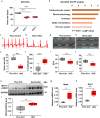
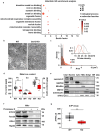
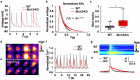
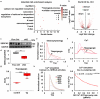
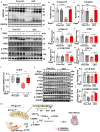
SYNTAXIN 12/13 (STX12), a member of the syntaxin protein family enriched in the brain and heart, plays important roles in vesicle recycling. Currently, the role of STX12 in cardiovascular physiology remains unclear. Using zebrafish and mice, it is shown that STX12 loss leads to pericardial edema, cardiac malformations, and heart failure. Stx12 depletion disrupts mitochondrial morphology, reduces iron and zinc levels, and impairs ATP production. Stx12-deficient cardiomyocytes exhibit prolonged repolarization due to decreased sarcoplasmic reticulum Ca2+-ATPase (SERCA) activity. Treatment with rapamycin, an mTOR inhibitor, restores mitochondrial protein expression and function by prompting the TFEB-PGC1α axis, enhances SERCA activity via the CAMKII-phospholamban pathway, and reduces the expression of stress markers. These findings suggest that STX12 plays an important role in the energy metabolism and metal homeostasis of cardiomyocytes. Enhancing mitochondrial function, autophagy, and SERCA activity through the administration of rapamycin may provide a potential therapeutic approach for cardiomyopathies associated with STX12 deficiency and hypometabolism.
Keywords: SERCA; Syntaxin 12/13; heart failure; mitochondria; rapamycin.
© 2025 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- King M., Kingery J., Casey B., Am. Fam. Physician 2012, 85, 1161. - PubMed
-
- Kemp C. D., Conte J. V., Cardiovasc. Pathol. 2012, 21, 365. - PubMed
-
- Tham Y. K., Bernardo B. C., Ooi J. Y. Y., Weeks K. L., McMullen J. R., Arch. Toxicol. 2015, 89, 1401. - PubMed
-
- Ruwhof C., van der Laarse A., Cardiovasc. Res. 2000, 47, 23. - PubMed
-
- Nakamura M., Sadoshima J., Nat. Rev. Cardiol. 2018, 15, 387. - PubMed
MeSH terms
Substances
Grants and funding
- 32071137/National Natural Science Foundation of China
- 92054103/National Natural Science Foundation of China
- 32000522/National Natural Science Foundation of China
- 32000855/National Natural Science Foundation of China
- ZYCXTD2023014/Funding for Scientific Research and Innovation Team of The First Affiliated Hospital of Zhengzhou University
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous
