Follicle on the Roof: Tertiary Lymphoid Structures in Central Nervous System Autoimmunity
- PMID: 40568975
- PMCID: PMC12199550
- DOI: 10.1111/imr.70045
Follicle on the Roof: Tertiary Lymphoid Structures in Central Nervous System Autoimmunity
Abstract
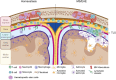
Leptomeningeal tertiary lymphoid structures (TLS) have emerged as a relatively common pathological feature of autoimmune disease, including multiple sclerosis (MS) and particularly in people with progressive and nonremitting MS. These ectopic lymphoid aggregates, observed in the leptomeninges adjacent to so-called "Type 3" sub-pial cortical lesions, are associated with more severe gray matter damage and worse clinical outcomes. Mouse models of MS that recapitulate TLS formation in the central nervous system (CNS) have provided critical insights into the mechanisms driving their development and maintenance. In these models of experimental autoimmune encephalomyelitis (EAE) initiation of TLS is facilitated by Th17 cells, which promote chronic inflammation via cytokines such as IL-17 and GM-CSF. The cell surface expression of lymphotoxin-α and lymphotoxin-β heterotrimers (LTαβ) on lymphocytes, including Th17 cells, elaborates the organization of ectopic lymphoid tissues via LTβR signaling on radio-resistant stromal cells and resident fibroblasts. Ultimately a pro-inflammatory environment characterized by cytokines such as IL-17 and GM-CSF promotes the recruitment of neutrophils which produce proteases and chemokines that sustain a pro-inflammatory milieu. Emerging EAE data suggest that disrupting TLS organization or targeting key pathways involved in their maintenance could represent promising strategies for modulating chronic CNS inflammation in MS. Understanding the cellular and molecular mechanisms regulating TLS dynamics is therefore critical for the development of therapies aimed at halting or reversing nonremitting MS disease.
Keywords: B cells; Th17; autoimmunity; experimental autoimmune encephalomyelitis; lymphotoxin; multiple sclerosis; neutrophils.
© 2025 The Author(s). Immunological Reviews published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Immune-responsive gene 1: The mitochondrial key to Th17 cell pathogenicity in CNS autoimmunity.Proc Natl Acad Sci U S A. 2025 Aug 12;122(32):e2427052122. doi: 10.1073/pnas.2427052122. Epub 2025 Aug 4. Proc Natl Acad Sci U S A. 2025. PMID: 40758870
-
Tissue-resident memory CD4+ T cells infiltrate the CNS in progressive multiple sclerosis and contribute to chronic autoimmunity in mice.Sci Transl Med. 2025 Jul 23;17(808):eadp8109. doi: 10.1126/scitranslmed.adp8109. Epub 2025 Jul 23. Sci Transl Med. 2025. PMID: 40700520
-
Sialomucin CD43 regulates T helper type 17 cell intercellular adhesion molecule 1 dependent adhesion, apical migration and transendothelial migration.Immunology. 2019 May;157(1):52-69. doi: 10.1111/imm.13047. Epub 2019 Feb 17. Immunology. 2019. PMID: 30690734 Free PMC article.
-
Tertiary lymphoid structures: chronic inflammatory microenvironments in kidney diseases.Int Immunol. 2025 Jul 22;37(8):445-455. doi: 10.1093/intimm/dxaf017. Int Immunol. 2025. PMID: 40127186 Review.
-
A novel immunological perspective on female-specific cancers: Exploring the signaling pathways of tertiary lymphoid structures and their clinical applications.Life Sci. 2025 Sep 15;377:123800. doi: 10.1016/j.lfs.2025.123800. Epub 2025 Jun 4. Life Sci. 2025. PMID: 40480623 Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

