Contrasting patterns in diversity and community assembly of bacterioplankton and three size fractions of protists in the South China Sea
- PMID: 40569084
- PMCID: PMC12285259
- DOI: 10.1128/aem.00436-25
Contrasting patterns in diversity and community assembly of bacterioplankton and three size fractions of protists in the South China Sea
Abstract
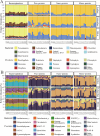
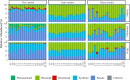
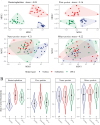
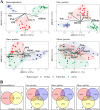
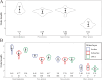
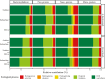
The microbial food web plays a critical role in marine ecosystems, composed of various cell sizes of microbial organisms. Here, high-throughput sequencing of the 16S and 18S rRNA genes was conducted to detect the community structure and distribution patterns of bacterioplankton (0.2 µm-2 µm) and three size fractions of protist communities, i.e., pico-protist (0.2 µm-2 µm), nano-protist (2 µm-20 µm), and micro-protist (20 µm-200 µm), in the euphotic zone of the South China Sea. The trophic mode compositions of protist communities varied significantly across three size fractions, characterized by a substantial prevalence of parasitic pico-protists (40% amplicon sequence variants) and a greater predominance of mixotrophic taxa within nano- and micro-protist communities. Furthermore, we detected stronger vertical stratification of bacterial and pico-protist communities, corresponding to the wider niche breadth of smaller cells and reliance on passive dispersal. Additionally, both bacterial and protist community assemblies were dominated by stochastic processes. The relative contribution of homogeneous selection in nano-protist community assembly was greater compared to other size fractions, probably related to high relative abundance of mixotrophs. In summary, our results suggest that both cell size and trophic mode affect marine microbial community assembly, and that neither the "size-plasticity" hypothesis nor the "size-dispersal" hypothesis fully matched microbial communities. Our analyses are important for a better understanding of the assemblage processes of marine epipelagic microbial communities and how they will respond to global change.IMPORTANCECell size is a key feature that influences microbial biology at both the cellular and community levels. Poorly understood is the extent to which diverse ecological factors influence the assembly of microbial communities of various sizes. Two important hypotheses addressing the mechanisms of biome assembly are "size-plasticity" and "size-dispersal." Here, we investigated epipelagic microbial communities to reveal differences in the ecological functions of various microbial sizes, to explore the association of ecological processes with niche and cell size, and to expand the current understanding of marine microbial community assemblages and their possible responses to future global change.
Keywords: South China Sea; community assembly; diversity; marine microbes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Sherr EB, Sherr BF. 2009. Food webs, microbial, p 174–189. In Schaechter M (ed), Encyclopedia of microbiology, 3rd ed. Academic Press, Oxford.
-
- Sieburth JM, Smetacek V, Lenz J. 1978. Pelagic ecosystem structure: heterotrophic compartments of the plankton and their relationship to plankton size fractions 1. Limnol Oceanogr 23:1256–1263. doi: 10.4319/lo.1978.23.6.1256 - DOI
-
- Sunagawa S, Acinas SG, Bork P, Bowler C, Tara Oceans Coordinators, Eveillard D, Gorsky G, Guidi L, Iudicone D, Karsenti E, Lombard F, Ogata H, Pesant S, Sullivan MB, Wincker P, de Vargas C. 2020. Tara Oceans: towards global ocean ecosystems biology. Nat Rev Microbiol 18:428–445. doi: 10.1038/s41579-020-0364-5 - DOI - PubMed
-
- Martiny AC, Hagstrom GI, DeVries T, Letscher RT, Britten GL, Garcia CA, Galbraith E, Karl D, Levin SA, Lomas MW, Moreno AR, Talmy D, Wang W, Matsumoto K. 2022. Marine phytoplankton resilience may moderate oligotrophic ecosystem responses and biogeochemical feedbacks to climate change. Limnol Oceanogr 67:S378–S389. doi: 10.1002/lno.12029 - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

