Interval Cytoreductive Surgery and Cisplatin- or Paclitaxel-Based HIPEC for Advanced Ovarian Cancer
- PMID: 40569595
- PMCID: PMC12203279
- DOI: 10.1001/jamanetworkopen.2025.17676
Interval Cytoreductive Surgery and Cisplatin- or Paclitaxel-Based HIPEC for Advanced Ovarian Cancer
Abstract
Importance: Ovarian cancer, often diagnosed at advanced stages, presents significant challenges in treatment and survival. Evaluation of different hyperthermic intraperitoneal chemotherapy (HIPEC) regimens could provide crucial insights to improve patient outcomes.
Objective: To evaluate whether HIPEC with paclitaxel (HIPEC-paclitaxel) is associated with similar oncological outcomes as HIPEC with cisplatin (HIPEC-cisplatin) in patients with advanced ovarian cancer undergoing interval cytoreductive surgery (iCRS).
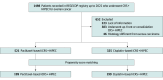
Design, setting, and participants: This multicenter retrospective cohort study included patients with advanced ovarian cancer who received iCRS and HIPEC. Patients with primary or secondary surgical procedures or nonovarian cancers were excluded. Data came from the National Registry of Peritoneal Carcinomatosis, which includes 27 Spanish specialized peritoneal oncology centers. Cases were included from January 2012 to December 2022. The study used propensity score matching to balance the groups and ensure comparability.
Exposure: HIPEC-cisplatin and HIPEC-paclitaxel, administered during iCRS. The HIPEC regimen was selected based on the standard clinical protocol for advanced ovarian cancer.
Main outcomes and measures: The primary end points were overall survival (OS) and disease-free survival (DFS). The secondary end point was the rate of complications in each group. These outcomes were predefined prior to data collection.
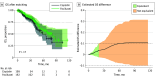
Results: A total of 846 patients (mean [SD] age, 59.04 [11.01] years) were included (325 [38.4%] in HIPEC-cisplatin group; 521 [61.6%] in HIPEC-paclitaxel group), and 199 patients in each group were propensity score matched. Among these 398 matched patients, the HIPEC-paclitaxel group had similar DFS and OS compared with the HIPEC-cisplatin group. Additionally, similar morbidity was observed. Equivalence in OS and DFS was observed during the initial 20 and 15 months of follow-up, respectively, with an equivalence margin of 0.1 respectively.
Conclusions and relevance: In this cohort study of patients with advanced ovarian cancer, HIPEC-paclitaxel was associated with comparable oncologic outcomes as HIPEC-cisplatin, suggesting that it could be a viable alternative. These findings support its use, especially in patients in whom cisplatin could be contraindicated. Further studies may help refine treatment protocols and improve patient-specific outcomes.
Conflict of interest statement
Figures

References
-
- Onda T, Satoh T, Ogawa G, et al. ; Japan Clinical Oncology Group . Comparison of survival between primary debulking surgery and neoadjuvant chemotherapy for stage III/IV ovarian, tubal and peritoneal cancers in phase III randomised trial. Eur J Cancer. 2020;130:114-125. doi: 10.1016/j.ejca.2020.02.020 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

