Multiphase OH Oxidation of Bisphenols: Chemical Transformation and Persistence in the Environment
- PMID: 40569786
- PMCID: PMC12243123
- DOI: 10.1021/acs.est.5c02030
Multiphase OH Oxidation of Bisphenols: Chemical Transformation and Persistence in the Environment
Abstract
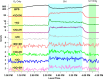
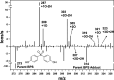
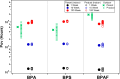
Bisphenol A (BPA) is a common endocrine disruptor widely found in commercial products. Despite negative human health effects, its usage is not fully banned worldwide with ongoing human exposure from sources including dust, aerosol particles, and surfaces. Although attention has been paid to the abundance of alternatives with similar structures that are replacing BPA, uncertainties remain with respect to their chemical transformations and products, toxicity, and environmental fate. We provide the first experimental and modeling assessment of gas-particle multiphase OH oxidation of BPA and six common bisphenol alternatives. We examine the transformation of condensed-phase BPA and its alternatives using an oxidation flow reactor with products monitored by online mass spectrometry. Fourteen products were identified and used to develop a generic mechanism applicable to all bisphenols and to provide inputs into an environmental fate model (PROduction-to-Exposure; PROTEX). Our modeling results highlight the role of heterogeneous surface reactions in determining the indoor retention of these chemicals and their relative environmental persistence indoors and outdoors. All investigated parent molecules yield transformation products predicted to accumulate indoors, with extended indoor persistence if a long chemical lifetime on surfaces (e.g., >100 weeks) is assumed. Evidence of phenoxy radical presence upon oxidation raises a human health risk concern.
Keywords: BPA alternatives; OH radical; environmental persistence; multiphase oxidation.
Figures


References
-
- Llevot A., Meier M. A. R.. Renewability - a Principle of Utmost Importance! Green Chem. 2016;18(18):4800–4803. doi: 10.1039/C6GC90087A. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

