TMPRSS2: A Key Host Factor in SARS-CoV-2 Infection and Potential Therapeutic Target
- PMID: 40569819
- PMCID: PMC12203450
- DOI: 10.4274/MMJ.galenos.2025.40460
TMPRSS2: A Key Host Factor in SARS-CoV-2 Infection and Potential Therapeutic Target
Abstract
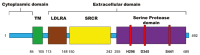
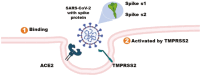
The transmembrane serine protease 2 (TMPRSS2) gene plays a crucial role in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection by priming the viral spike protein for membrane fusion and facilitating viral entry into host cells. This review aims to explore the molecular function of TMPRSS2, its genetic variations, and its potential as a therapeutic target in corona virus disease 2019 (COVID-19) and other respiratory viral infections. TMPRSS2 is highly expressed in lung and prostate tissues and is regulated by androgens, which may contribute to sex-based differences in COVID-19 severity. Genetic polymorphisms in TMPRSS2 have been been associated with variability in disease susceptibility and severity across populations. Several TMPRSS2 inhibitors, including serine protease inhibitors, such as camostat mesylate and nafamostat, have demonstarted promise in blocking viral entry. In addition, RNA based strategies such as siRNA and clustered regularly interspaced short palindromic repeats offer potential approaches for downregulating TMPRSS2 expression. However, the development of selective inhibitors that avoid off target effects remains a challenge. The presence of TMPRSS2-ERG gene fusion, commonly found in prostate cancer, has also been linked to altered COVID-19 susceptibility, suggesting a complex interplay between viral infection and cancer biology. This review also discusses future perspectives, including large-scale genomic studies to identify high-risk individuals, the development of next-generation TMPRSS2 inhibitors, and potential broad-spectrum antiviral therapies targeting TMPRSS2.
Transmembran serin proteaz 2 (TMPRSS2) geni, membran füzyonu için viral spike proteinini hazırlayarak ve konakçı hücrelere viral girişi kolaylaştırarak şiddetli akut solunum yolu sendromu koronavirüs 2 (SARS-CoV-2) enfeksiyonunda önemli bir rol oynar. Bu derleme, TMPRSS2’nin moleküler işlevini, genetik varyasyonlarını ve koronavirüs hastalığı 2019 (COVID-19) ve diğer solunum yolu viral enfeksiyonlarında terapötik bir hedef olarak potansiyelini araştırmayı amaçlamaktadır. TMPRSS2 akciğer ve prostat dokularında yüksek oranda eksprese edilir ve androjenler tarafından düzenlenir, bu da COVID-19 şiddetinde cinsiyete dayalı farklılıklara katkıda bulunabilir. TMPRSS2’deki genetik polimorfizmler, popülasyonlar arasında hastalık duyarlılığı ve şiddetindeki değişkenlikle ilişkilendirilmiştir. Camostat mesilat ve nafamostat gibi serin proteaz inhibitörleri de dahil olmak üzere çeşitli TMPRSS2 inhibitörleri, viral girişin bloke edilmesinde umut vaat etmiştir. Buna ek olarak, siRNA ve kümelenmiş düzenli aralıklı kısa palindromik tekrarlar gibi RNA tabanlı stratejiler, TMPRSS2 ekspresyonunun aşağı regülasyonu için potansiyel yaklaşımlar sunmaktadır. Bununla birlikte, hedef dışı etkilerden kaçınan seçici inhibitörlerin geliştirilmesi bir zorluk olmaya devam etmektedir. Prostat kanserinde yaygın olarak bulunan TMPRSS2-ERG gen füzyonunun varlığı da değişmiş COVID-19 duyarlılığı ile bağlantılıdır ve viral enfeksiyon ile kanser biyolojisi arasında karmaşık bir etkileşim olduğunu düşündürmektedir. Bu derlemede ayrıca, yüksek riskli bireyleri belirlemek için büyük ölçekli genomik çalışmalar, yeni nesil TMPRSS2 inhibitörlerinin geliştirilmesi ve TMPRSS2’yi hedefleyen potansiyel geniş spektrumlu antiviral tedaviler de dahil olmak üzere geleceğe yönelik perspektifler tartışılmaktadır.
Keywords: Severe acute respiratory syndrome coronavirus 2; Transmembrane serine protease 2; corona virus disease-2019; therapeutic target; viral entry.
Copyright© 2025 The Author. Published by Galenos Publishing House on behalf of Istanbul Medeniyet University Faculty of Medicine.
Conflict of interest statement
Conflict of Interest: The authors have no conflict of interest to declare.
Figures

Similar articles
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
-
C-Terminal Analogues of Camostat Retain TMPRSS2 Protease Inhibition: New Synthetic Directions for Antiviral Repurposing of Guanidinium-Based Drugs in Respiratory Infections.Int J Mol Sci. 2025 Jul 15;26(14):6761. doi: 10.3390/ijms26146761. Int J Mol Sci. 2025. PMID: 40725007 Free PMC article.
-
COVID-19 management in patients with comorbid conditions.World J Virol. 2025 Jun 25;14(2):102674. doi: 10.5501/wjv.v14.i2.102674. World J Virol. 2025. PMID: 40575645 Free PMC article. Review.
-
Determinants of susceptibility to SARS-CoV-2 infection in murine ACE2.J Virol. 2025 Jun 17;99(6):e0054325. doi: 10.1128/jvi.00543-25. Epub 2025 May 12. J Virol. 2025. PMID: 40353671 Free PMC article.
-
The crystal structure of coronavirus RBD-TMPRSS2 complex provides basis for the discovery of therapeutic antibodies.Nat Commun. 2025 Jul 18;16(1):6636. doi: 10.1038/s41467-025-62023-2. Nat Commun. 2025. PMID: 40681508 Free PMC article.
References
-
- Reis S, Faske A, Monsef I, et al. Anticoagulation in COVID-19 patients - an updated systematic review and meta-analysis. Thromb Res. 2024;238:141–150. - PubMed
-
- Features, evaluation, and treatment of coronavirus (COVID-19) 2025 - PubMed
-
- Amara A, Trabelsi S, Hai A, Zaidi SHH, Siddiqui F, Alsaeed S. Equivocating and deliberating on the probability of covid-19 infection serving as a risk factor for lung cancer and common molecular pathways serving as a link. Pathogens. 2024;13(12):1070. doi: 10.3390/pathogens13121070. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
