Modelling the impact of initiation delay, duration and prior PrEP on the efficacy of post-exposure prophylaxis containing a tenofovir/emtricitabine backbone
- PMID: 40569890
- PMCID: PMC12231637
- DOI: 10.1002/jia2.26454
Modelling the impact of initiation delay, duration and prior PrEP on the efficacy of post-exposure prophylaxis containing a tenofovir/emtricitabine backbone
Abstract
Introduction: Pre- and post-exposure prophylaxis (PrEP and PEP) are important pillars of the HIV prevention portfolio to reduce the risk of acquisition just before or after HIV exposure. While PrEP efficacy has been elucidated in many randomized clinical trials, corresponding data for PEP is extremely difficult to obtain in a controlled setting. Consequently, it is almost impossible to study the impact of PEP initiation delay and duration on HIV risk reduction clinically, which would inform recommendations on PEP use.
Methods: We employ pharmacokinetics, pharmacodynamics and viral dynamics models, along with individual factors, such as drug adherence to investigate the impact of initiation delay and PEP duration on HIV risk reduction. We evaluated PEP using two- and three-drug regimens with a TDF/FTC backbone. Moreover, we study PEP efficacy in the context of PrEP-to-PEP transitions.
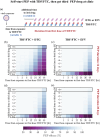
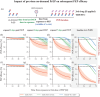
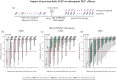
Results: In our simulations, early initiation of PEP emerged as a pivotal factor for HIV risk reduction. We found that 2-drug (TDF/FTC) PEP may insufficiently protect when initiated > 1 hour post-exposure. When adding a third drug, early initiation was still a critical factor; however, over 90% efficacy could be achieved when PEP was initiated 48 hours post-exposure and taken for at least 14-28 days, depending on the efficacy of the third-drug component. When investigating PrEP-PEP transitions, we observed that preceding PrEP can (1) contribute directly to prophylactic efficacy, and (2) boost subsequent PEP efficacy by delaying initial viral dynamics and building-up drug concentrations, overall facilitating self-managed transitioning between PrEP and PEP.
Conclusions: Our study confirms the critical role of early (< 48 hours) PEP initiation, preferably with three drugs taken for 28 days. Self-start with TDF/FTC and later addition of a third drug is better than not self-starting. Furthermore, our study highlights the synergy between recent PrEP intake and PEP and may help to inform recommendations on PEP use.
Keywords: HIV; TDF/FTC; mathematical modelling; post‐exposure prophylaxis; pre‐exposure prophylaxis; quantitative systems pharmacology.
© 2025 The Author(s). Journal of the International AIDS Society published by John Wiley & Sons Ltd on behalf of International AIDS Society.
Conflict of interest statement
JF received research funding from GSK for a shingles vaccine study. The remaining authors declare no competing interests.
Figures


References
-
- UNAIDS . Global HIV and AIDS statistics — 2023 fact sheet. 2023.
-
- Eisinger RW, Dieffenbach CW, Fauci AS. HIV viral load and transmissibility of HIV infection: undetectable equals untransmittable. JAMA. 2019;321:451–2. - PubMed
-
- Landovitz RJ, Scott H, Deeks SG. Prevention, treatment and cure of HIV infection. Nat Rev Micro. 2023;21:657–70. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

