Genome-wide association study uncovers a novel gene responsible for rice seedling submergence tolerance
- PMID: 40569892
- PMCID: PMC12392957
- DOI: 10.1111/pbi.70187
Genome-wide association study uncovers a novel gene responsible for rice seedling submergence tolerance
Abstract
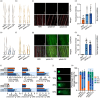
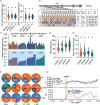
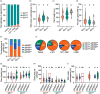
Submergence tolerance is crucial for the direct seeding of rice, yet long-term domestication and breeding have inadvertently reduced the adaptability of cultivated rice to submergence stress. Here, we identify a nucleic acid excision repair protein-encoding gene qSHS5 as an essential regulator of seedling height under submergence through a genome-wide association study in 322 rice accessions. Disruption of qSHS5 in mutants resulted in seedling growth inhibition under submergence, while growth remains comparable to wild-type under normal conditions. This inhibition is primarily due to decreased cell number resulting from G1 phase cell cycle arrest. Further investigation showed that levels of reactive oxygen species (ROS), O2 - and H2O2 significantly increased, and DNA damage was aggravated in qshs5 mutants under submergence. Additionally, we find the submergence-tolerant haplotype qSHS5H4 has been progressively lost, while the elite haplotype qSHS5H3 has been largely overlooked during the breeding of semi-dwarf and high-yield in rice. Importantly, we demonstrate that combining qSHS5H3 with the semi-dwarfing haplotype SD1H1 exhibited high yield without compromising submergence tolerance, offering significant potential for future breeding programmes targeting direct seeding cultivation. This study not only identifies a novel superior allele but also provides valuable insights for future improvement of rice cultivation, particularly under climate change-induced submergence for direct seeding.
Keywords: ROS; breeding improvement; natural variation; qSHS5; rice; submergence tolerance.
© 2025 The Author(s). Plant Biotechnology Journal published by Society for Experimental Biology and The Association of Applied Biologists and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Identification of submergence tolerance QTLs/genes during seed germination in 432 rice varieties by GWAS.BMC Plant Biol. 2025 Sep 1;25(1):1172. doi: 10.1186/s12870-025-07269-0. BMC Plant Biol. 2025. PMID: 40890607
-
Differential adaptations of japonica rice to submergence stress during the tillering stage under various seedling cultivation and transplanting methods.Front Plant Sci. 2025 Jun 19;16:1607055. doi: 10.3389/fpls.2025.1607055. eCollection 2025. Front Plant Sci. 2025. PMID: 40612614 Free PMC article.
-
Combining QTL mapping and transcriptomics to identify candidate genes for cold tolerance during the budding and seedling stages in rice.BMC Genomics. 2025 Aug 19;26(1):756. doi: 10.1186/s12864-025-11937-8. BMC Genomics. 2025. PMID: 40830828 Free PMC article.
-
Meta-Analysis of Iron Excess Stress in Rice: Genes and Mechanisms of Tolerance to Acidic Soil.Physiol Plant. 2025 Sep-Oct;177(5):e70473. doi: 10.1111/ppl.70473. Physiol Plant. 2025. PMID: 40873056 Free PMC article. Review.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
References
-
- Ahmed, M.B. , Alghamdi, A.A.A. , Islam, S.U. , Ahsan, H. and Lee, Y.S. (2023) The complex roles of DNA repair pathways, unhibitors, hyperthermia, and contact inhibition in cell cycle halts. Mini‐Rev. Med. Chem. 23, 514–529. - PubMed
-
- Basu, S. , Kumari, S. , Kumar, P. , Kumar, G. and Rajwanshi, R. (2021) Redox imbalance impedes photosynthetic activity in rice by disrupting cellular membrane integrity and induces programmed cell death under submergence. Physiol. Plant. 172, 1764–1778. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

