SHR-A1811, a novel anti-HER2 antibody-drug conjugate with optimal drug-to-antibody ratio, efficient tumor killing potency, and favorable safety profiles
- PMID: 40569929
- PMCID: PMC12200682
- DOI: 10.1371/journal.pone.0326691
SHR-A1811, a novel anti-HER2 antibody-drug conjugate with optimal drug-to-antibody ratio, efficient tumor killing potency, and favorable safety profiles
Abstract
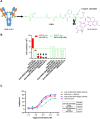
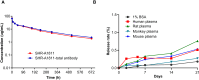
HER2-targeting antibody-drug conjugates (ADCs), especially trastuzumab deruxtecan (T-DXd), have revolutionized the treatment landscape of HER2-expressing or mutant cancers. However, undesired adverse events are still inevitable and it is necessary to discover a HER2-directed ADC with better safety profiles. SHR-A1811 is composed of trastuzumab, a cleavable linker and a novel topoisomerase I inhibitor, SHR169265. The results indicated that SHR169265 shows better permeability, strong cytotoxicity and faster systemic clearance than DXd analog (SHR197971). The drug-to-antibody ratio (DAR) of SHR-A1811 was optimized as 6 via balancing efficacy and toxicity. SHR-A1811 showed HER2-dependent growth inhibition against various cell lines and desirable bystander killing capability. SHR-A1811 led to tumor growth inhibition or even regression in a dose-dependent manner, at least comparable as HRA18-C015 (a synthesized T-DXd) and anti-HER2-SHR169265 (DAR 8) in multiple mouse xenograft models with a range of HER2 expression levels. SHR-A1811 exhibited a good pharmacokinetics profile, outstanding stability in plasma across different species and a favorable preclinical safety profile. The highest non-severely toxic dose (HNSTD) in cynomolgus monkeys was 40 mg/kg with thymus as the main target organ. The above results suggested that SHR-A1811 is a potential best-in-class anti-HER2 ADC with a highly permeable payload, optimized DAR, great potency and better safety profiles. Currently SHR-A1811 has entered phase II and phase III clinical studies for breast cancer, gastric cancer, colorectal cancer, and NSCLC.
Copyright: © 2025 Zhang et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
This study was funded by Shanghai Hengrui Pharmaceutical Co., Ltd., a leading company in China focuses on new drug development for multiple diseases to address unmet medical needs. SHR-A1811 is developed by Shanghai Hengrui and multiple phase II and III clinical trials are being conducted. The result of a global phase I trial has been published in Journal of Clinical Oncology. The authors, Ting Zhang, Jianyan Xu, Junzhao Yin, Yun Gao, Hanwen Zheng, Beibei Fu, Jiakang Sun, Zhibin Xu, Shiwei Tu, Yuchang Mao, Weiyun Wen, Bolei Qu, Lingfeng You, Zhendong Xue, Dan Cao, Jun Feng, Min Hu, and Feng He are paid employees of Shanghai Hengrui Pharmaceutical Co., Ltd. Please note this does not alter our adherence to PLOS ONE policies on sharing data and materials, and the funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Figures


References
-
- Keam SJ. Trastuzumab deruxtecan: First approval. Drugs. 2020;80(5):501–8. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

