Investigating immune amnesia after measles virus infection in two West African countries: A study protocol
- PMID: 40569945
- PMCID: PMC12200839
- DOI: 10.1371/journal.pone.0314828
Investigating immune amnesia after measles virus infection in two West African countries: A study protocol
Abstract
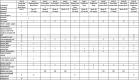
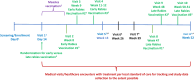
"Investigation of Immune Amnesia Following Measles Infection in Select African Regions" (ClinicalTrials.gov Identifier: NCT06153979) is a prospective, observational, longitudinal study being conducted in two West African countries; Guinea and Mali. Acute measles virus (MeV) infection has been shown to result in a loss of pre-existing immunity (immune amnesia). MeV-induced immune amnesia has not been studied in West Africa where continual MeV outbreaks occur. Additionally previous studies have relied on naturally occurring exposures to viruses to examine the immune systems ability to create antibodies. Thus, the overall goal of this protocol is to investigate the impact of MeV infection on pre-existing immunity to endemic pathogens in West Africa, observe the effect of a subsequent exposure to a novel pathogen (rabies vaccine), and measure the frequency of subsequent healthcare visits. A total of 256 children aged 1-15 years are being enrolled into one of two study arms: those with acute MeV infection (cases) as confirmed by laboratory testing and without (controls). Controls must be immune to MeV (have IgG). Blood samples are collected at multiple time points including screening (Day 0), at an optional visit to repeat IgM serology for inconclusive or negative Day 0 results (Day 7-10), and during follow-up visits on Day 14, Week 13, and Week 52. These blood samples will be tested to evaluate both humoral and cellular immune responses to a panel of viruses, bacteria, and parasites, including pathogens endemic to West Africa. To explore how recent MeV infection may affect the child's ability to respond to a new exposure, all participants will receive a rabies vaccine (as a controlled stimulus) at one of two timepoints post Day 0 visit. Biological samples will be collected after vaccination to assess if the rabies vaccine response differs: 1) between cases and controls, and 2) based on the time since acute MeV infection. In addition, the study team will collect information on healthcare encounters during the year-long follow-up to determine if there is a difference in the number of encounters by study group. The findings of this study will further the understanding of the MeV immune amnesia phenotype by understanding its impact on endemic pathogens and subsequent immune response following infection.
Copyright: This is an open access article, free of all copyright, and may be freely reproduced, distributed, transmitted, modified, built upon, or otherwise used by anyone for any lawful purpose. The work is made available under the Creative Commons CC0 public domain dedication.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Vittrup DM, Laursen ACL, Malon M, Soerensen JK, Hjort J, Buus S, et al. Measles-mumps-rubella vaccine at 6 months of age, immunology, and childhood morbidity in a high-income setting: study protocol for a randomized controlled trial. Trials. 2020;21(1):1015. doi: 10.1186/s13063-020-04845-7 - DOI - PMC - PubMed
-
- WHO. WHO. Measles fact sheet. 2023. Accessed 2024 February 7. https://www.who.int/en/news-room/fact-sheets/detail/measles
-
- WHO. A 30-fold rise of measles cases in 2023 in the WHO European region warrants urgent action. 2023. https://www.who.int/europe/news/item/14-12-2023-a-30-fold-rise-of-measle...
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

