The Hippo-YAP/β-catenin signaling axis coordinates odontogenic differentiation in dental pulp stem cells: Implications for dentin-pulp regeneration
- PMID: 40570025
- PMCID: PMC12200642
- DOI: 10.1371/journal.pone.0326978
The Hippo-YAP/β-catenin signaling axis coordinates odontogenic differentiation in dental pulp stem cells: Implications for dentin-pulp regeneration
Abstract
Objective: This study investigated the interplay between Hippo-YAP and β-catenin signaling in regulating odontogenic differentiation of human dental pulp stem cells (DPSCs) and explored its potential implications for dentin-pulp regeneration.
Methods: Using lentivirus-mediated YAP overexpression/silencing, β-catenin siRNA knockdown, and pharmacological Wnt inhibition (via WIF-1), we assessed DPSC proliferation, migration, mineralization, and molecular markers (via qRT-PCR, immunofluorescence). In vivo validation employed subcutaneous transplantation of DPSC-seeded scaffolds in immunocompromised mice.
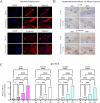
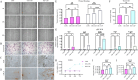
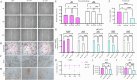
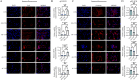
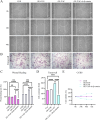
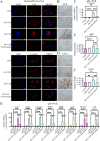
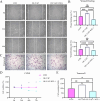
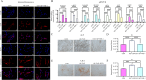
Results: YAP activation enhanced DPSC proliferation (1.44-fold), migration (1.39-fold), invasion (1.54-fold), and differentiation, as evidenced by elevated ALP activity (1.46-fold) and mineralization (1.36-fold). We observed transcriptional upregulation of odontogenic markers (RUNX2, DSPP, DMP1, OCN, ALP) and Wnt pathway components (β-catenin, Cyclin D1, c-Myc). Immunofluorescence revealed coordinated YAP and β-catenin expression patterns during differentiation. β-catenin silencing or Wnt inhibition abolished YAP-mediated functional enhancements and simultaneously suppressed YAP expression, partially confirming bidirectional regulation. In vivo, YAP-overexpressing DPSCs exhibited 1.27- to 1.62-fold induction of dentin-specific markers and β-catenin, whereas YAP silencing impaired these markers expression.
Conclusions: Our findings demonstrate that coordinated YAP and β-catenin signaling drives DPSC odontogenesis, with potential implications for dentin regeneration. Although reciprocal regulation is evident, direct molecular interactions require further validation.
Copyright: © 2025 Chen et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

