Discovery of functional factorless internal ribosome entry site-like structures through virome mining
- PMID: 40570062
- PMCID: PMC12221177
- DOI: 10.1371/journal.ppat.1013255
Discovery of functional factorless internal ribosome entry site-like structures through virome mining
Abstract
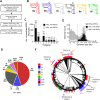
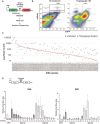
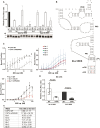
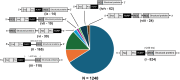
All viruses must co-opt the host translational machinery for viral protein synthesis. The dicistrovirus intergenic region internal ribosome entry site (IGR-IRES) utilizes the most streamlined translation mechanism by adopting a triple pseudoknot structure that directly recruits and binds within the intersubunit space of the ribosome and initiates translation from a non-AUG codon. The origin of this unprecedented mechanism is not known. Using a bioinformatics pipeline to examine the diversity and function of IRESs across RNA viromes, we searched for IRES-like RNA structures using RNA covariance models for multiple IRES sub-types, and tested functional IRES by using a dual-fluorescent lentiviral library reporter screen. We identified over >4,700 dicistro-like genomes with ~32% containing putative IRES structures, including novel viral genome arrangements with multiple IRESs and IRESs embedded within open-reading frames (ORFs). Predicted IRESs bound directly to purified ribosomes and supported internal ribosome entry activity in vitro and in vivo. Moreover, internal IRESs embedded within an ORF of monocistronic genomes were functional and operated simultaneously to produce the downstream ORF. We also identified IRES-like structures within non-dicistrovirus viral genomes, including in the families Tombusviridae and Narnaviridae that bound to ribosomes directly and a subset can direct internal ribosome entry. This study provides a framework to map the origin of factorless IRES mechanisms and study the diverse viral strategies utilizing RNA-based mechanisms.
Copyright: © 2025 Chapagain et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




Similar articles
-
Factor-Dependent Internal Ribosome Entry Site and -1 Programmed Frameshifting Signal in the Bemisia-Associated Dicistrovirus 2.Viruses. 2024 Apr 28;16(5):695. doi: 10.3390/v16050695. Viruses. 2024. PMID: 38793577 Free PMC article.
-
The Hinge Region of the Israeli Acute Paralysis Virus Internal Ribosome Entry Site Directs Ribosomal Positioning, Translational Activity, and Virus Infection.J Virol. 2022 Mar 9;96(5):e0133021. doi: 10.1128/JVI.01330-21. Epub 2022 Jan 12. J Virol. 2022. PMID: 35019716 Free PMC article.
-
A circular split nanoluciferase reporter for validating and screening putative internal ribosomal entry site elements.RNA. 2024 Oct 16;30(11):1529-1540. doi: 10.1261/rna.080008.124. RNA. 2024. PMID: 39103230
-
Commandeering the Ribosome: Lessons Learned from Dicistroviruses about Translation.J Virol. 2016 May 27;90(12):5538-5540. doi: 10.1128/JVI.00737-15. Print 2016 Jun 15. J Virol. 2016. PMID: 27053555 Free PMC article. Review.
-
Mechanism of translation initiation by Dicistroviridae IGR IRESs.Virology. 2011 Mar 15;411(2):355-61. doi: 10.1016/j.virol.2011.01.005. Epub 2011 Feb 1. Virology. 2011. PMID: 21284991 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

