Mechanistic Elucidation of Tricholoma mongolicum Polysaccharides in Treating MAFLD via Regulation of the Gut Microbiota-Metabolite-Ferroptosis Axis: A Multi-Omics Perspective
- PMID: 40570246
- PMCID: PMC12257537
- DOI: 10.1021/acs.jafc.5c05877
Mechanistic Elucidation of Tricholoma mongolicum Polysaccharides in Treating MAFLD via Regulation of the Gut Microbiota-Metabolite-Ferroptosis Axis: A Multi-Omics Perspective
Abstract
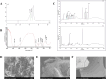
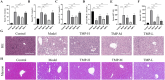
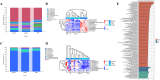
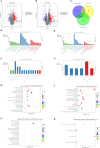
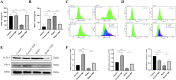
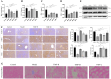
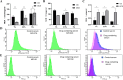
This study aimed to elucidate the modulatory effects and underlying molecular mechanisms of Tricholoma mongolicum polysaccharide (TMP) in the context of metabolic dysfunction-associated fatty liver disease (MAFLD). High-performance gel permeation chromatography (HPGPC) analysis indicated a bimodal molecular weight distribution. Monosaccharide composition profiling revealed a predominance of glucose and galactose among other constituents. Scanning electron microscopy (SEM) illustrated a porous, aggregated colloidal microstructure. In a model of MAFLD, TMP intervention significantly attenuated serum levels of TC, TG, and AST, ALT, accompanied by notable histological improvements, including reduced hepatic steatosis and inflammatory cell infiltration. Metagenomic analysis demonstrated that TMP substantially enhanced gut microbial α-diversity, restructured microbial community composition, decreased the Firmicutes/Bacteroidetes ratio, enriched SCFAs-producing genera, and suppressed the excessive proliferation of pro-inflammatory bacterial genera. Integrated proteomic and lipidomic analyses revealed that TMP inhibited hepatic immune-inflammatory responses and ferroptosis pathways, enhanced pathways associated with metabolic homeostasis. Furthermore, TMP modulated hepatic iron metabolism by upregulating the Nrf2/GPx4 antioxidant axis and FPN1 while downregulating TFR1, thereby alleviating oxidative stress and iron overload. These findings demonstrate that TMP exerts therapeutic efficacy through a bidirectional gut-liver regulatory mechanism involving microbial modulation, ferroptosis inhibition, metabolic reprogramming, and activation of antioxidant defenses. This research provides novel insights and molecular targets for the development of natural polysaccharide-based interventions for MAFLD.
Keywords: Tricholoma mongolicum polysaccharide; bidirectional gut−liver axis regulation; ferroptosis; gut microbiota; metabolic dysfunction-associated fatty liver disease; multiomics.
Figures




Similar articles
-
Multiomics reveals metformin's dual role in gut microbiome remodeling and hepatic metabolic reprogramming for MAFLD intervention.Sci Rep. 2025 Jul 2;15(1):22699. doi: 10.1038/s41598-025-07557-7. Sci Rep. 2025. PMID: 40594788 Free PMC article.
-
Lactobacillus plantarum 1-2-3 inhibits ferroptosis by regulating dysregulated fatty acid metabolism to protect mice from high-fat diet-induced MAFLD.Free Radic Biol Med. 2025 Oct;238:137-151. doi: 10.1016/j.freeradbiomed.2025.06.042. Epub 2025 Jun 22. Free Radic Biol Med. 2025. PMID: 40555343
-
Obacunone ameliorates high-fat diet-induced MAFLD by regulating the PPARγ-FABP1/CD36 axis and the gut-liver crosstalk.Phytomedicine. 2025 Aug 18;147:157180. doi: 10.1016/j.phymed.2025.157180. Online ahead of print. Phytomedicine. 2025. PMID: 40850071
-
Advances in the study of oral microbiota and metabolism associated fatty liver disease: a systematic review.Front Cell Infect Microbiol. 2024 Nov 12;14:1491696. doi: 10.3389/fcimb.2024.1491696. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 39600870 Free PMC article.
-
Gut microbiota and metabolomics in metabolic dysfunction-associated fatty liver disease: interaction, mechanism, and therapeutic value.Front Cell Infect Microbiol. 2025 Jul 23;15:1635638. doi: 10.3389/fcimb.2025.1635638. eCollection 2025. Front Cell Infect Microbiol. 2025. PMID: 40771314 Free PMC article. Review.
References
-
- Kozumi K., Kodama T., Murai H., Sakane S., Govaere O., Cockell S., Motooka D., Kakita N., Yamada Y., Kondo Y., Tahata Y., Yamada R., Hikita H., Sakamori R., Kamada Y., Daly A. K., Anstee Q. M., Tatsumi T., Morii E., Takehara T.. Transcriptomics Identify Thrombospondin-2 as a Biomarker for NASH and Advanced Liver Fibrosis. Hepatology. 2021;74:2452–2466. doi: 10.1002/hep.31995. - DOI - PMC - PubMed
-
- Kumar A., Sharma A., Duseja A., Das A., Dhiman R. K., Chawla Y. K., Kohli K. K., Bhansali A.. Patients with Nonalcoholic Fatty Liver Disease (NAFLD) have Higher Oxidative Stress in Comparison to Chronic Viral Hepatitis. J. Clin. Exp. Hepatol. 2013;3:12–18. doi: 10.1016/j.jceh.2012.10.009. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

