Role of paternal Oryza sativa Baby Booms (OsBBMs) in initiating de novo gene expression and regulating early zygotic development in rice
- PMID: 40570546
- PMCID: PMC12201997
- DOI: 10.1111/tpj.70305
Role of paternal Oryza sativa Baby Booms (OsBBMs) in initiating de novo gene expression and regulating early zygotic development in rice
Abstract
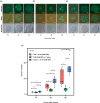
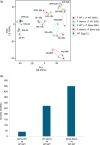
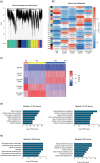
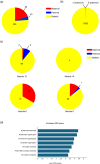
Oryza sativa BABY BOOM 1 (OsBBM1), a member of the AP2/ERF family of transcription factors, is expressed from paternal allele in rice zygote and plays a crucial role in initiating zygotic development. However, the mechanism how the paternal OsBBM1 drives this development remains unclear. Rice zygotes with four different gamete combinations with or without functional paternal OsBBMs were produced by electrofusion, using gametes isolated from bbms triple mutants and wild-type rice plants. Developmental and gene expression profiles of these types of zygotes were intensively analyzed and compared. Mutations in OsBBM1, OsBBM2, and OsBBM3 on the paternal alleles caused developmental arrest or delay in the zygotes, while defects in OsBBMs on the maternal allele had minimal effects on zygotic development. Paternal allele of OsBBMs significantly influenced gene expression profiles related to regulation of basic cellular processes, such as chromosome/chromatin organization/assembly and cell cycle/division compared to the maternal allele of OsBBMs. Majority of these genes were upregulated in zygotes from paternal/parental alleles via paternal OsBBMs. Paternal OsBBMs initiate early development of rice zygotes through the regulation of expression profiles of genes controlling status of chromosome/chromatin and cell cycle/division.
Keywords: AP2 transcription factor; Oryza sativa; OsBBMs; development; fertilization; paternal allele; transcriptome analysis; zygote.
© 2025 The Author(s). The Plant Journal published by Society for Experimental Biology and John Wiley & Sons Ltd.
Conflict of interest statement
The authors have not declared a conflict of interest.
Figures



Similar articles
-
Expression of Genes from Paternal Alleles in Rice Zygotes and Involvement of OsASGR-BBML1 in Initiation of Zygotic Development.Plant Cell Physiol. 2019 Apr 1;60(4):725-737. doi: 10.1093/pcp/pcz030. Plant Cell Physiol. 2019. PMID: 30801122
-
Ethylenediurea (EDU) inhibits OsORAP1 expression in rice (Oryza sativa L:): Varietal differences in ozone protection efficacy.PLoS One. 2025 Jul 2;20(7):e0327162. doi: 10.1371/journal.pone.0327162. eCollection 2025. PLoS One. 2025. PMID: 40601707 Free PMC article.
-
The H1/H5 domain contributes to OsTRBF2 phase separation and gene repression during rice development.Plant Cell. 2024 Sep 3;36(9):3787-3808. doi: 10.1093/plcell/koae199. Plant Cell. 2024. PMID: 38976557 Free PMC article.
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer.Health Technol Assess. 2001;5(32):1-195. doi: 10.3310/hta5320. Health Technol Assess. 2001. PMID: 12065068
References
-
- Boycheva, I. , Vassileva, V. , Revalska, M. , Zehirov, G. & Iantcheva, A. (2015) Cyclin‐like F‐box protein plays a role in growth and development of the three model species Medicago truncatula, Lotus japonicus, and Arabidopsis thaliana . Research and Reports in Biology, 6, 117–130.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

