Integrating genetic polymorphisms and clinical data to develop predictive models for skin toxicity in breast cancer radiation therapy
- PMID: 40570703
- PMCID: PMC12264628
- DOI: 10.1016/j.breast.2025.104506
Integrating genetic polymorphisms and clinical data to develop predictive models for skin toxicity in breast cancer radiation therapy
Abstract
Background: We aim to develop and validate predictive models for acute and late skin toxicity in breast cancer (BC) patients undergoing radiation therapy (RT). Models incorporate a genetic profile-comprising candidate single nucleotide polymorphisms (SNPs) in non-coding RNAs and previously reported toxicity-associated variants-combined with clinical variables.
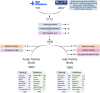
Methods: The study involved 1979 BC patients monitored for two to eight years post-RT in a multi-centre study. We assessed acute (oedema/erythema) and late (atrophy/fibrosis) toxicity using logistic regression and Cox proportional hazards models. The cohort was divided into training and validation datasets.
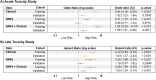
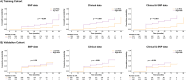
Results: Six SNPs demonstrated to be predictors of acute (rs13116075, rs12565978, rs72550778 and rs7284767) and late toxicity (rs16837908 and rs61764370) either in the training or validation cohort. However, none of these SNPs were consistently associated with toxicity across both stages of analysis. The rs13116075, rs12565978 and rs16837908 were previously reported to be associated with RT toxicity. In the validation phase, SNP-based models showed limited predictive ability, with AUC values of 0.49 and c-index of 0.54 for acute and late toxicity, respectively. Models incorporating either clinical variables alone or in combination with SNPs achieved similar AUC and c-index values of ∼0.60 for acute and late toxicity, respectively. However, the combined model exhibited the highest predictive accuracy for acute and late toxicity, both in the training and the validation cohorts.
Conclusions: Our findings highlight the importance of combining clinical data with genetic markers to enhance the accuracy of models predicting RT toxicity in BC.
Keywords: Breast cancer; Literature candidate polymorphisms' validation; Non-coding RNA polymorphisms; Predictive models; Radiation therapy-induced side-effects.
Copyright © 2025 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare no conflict of interest.
Figures



References
-
- Darby S., McGale P., Correa C., Taylor C., Arriagada R., Clarke M., et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10 801 women in 17 randomised trials. Lancet. 2011;378:1707–1716. doi: 10.1016/S0140-6736(11)61629-2. - DOI - PMC - PubMed
-
- Xu L., Osei B., Osei E. A review of radiation genomics: integrating patient radiation response with genomics for personalised and targeted radiation therapy. J Radiother Pract. 2019;18:198–209. doi: 10.1017/S1460396918000547. - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

