Microcurrent stimulation induces cell death in p53-mutant and 5-FU-resistant breast cancer
- PMID: 40570957
- PMCID: PMC12301737
- DOI: 10.1016/j.jbc.2025.110414
Microcurrent stimulation induces cell death in p53-mutant and 5-FU-resistant breast cancer
Abstract
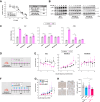
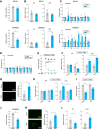
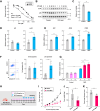
5-Fluorouracil (5-FU) is a commonly used chemotherapeutic agent for breast cancer. Its efficacy relies on the function of p53, and mutations in p53 contribute to the development of resistance during 5-FU chemotherapy. Here, we report that microcurrent stimulation (MCS) of a p53-mutant breast cancer cell line induces p53-mediated cell death. Although MDA-MB-231 and MDA-MB-468 cells, both human breast cancer cell lines, are less sensitive to 5-FU due to p53 mutations, MCS (300 μA for 30 min) induced apoptosis in these cells and improved the antitumor effect of 5-FU in tumor-bearing mice. MCS-induced apoptosis was mediated by an increase in intracellular Cu2+ ions and reactive oxygen species, along with the concurrent transcriptional enhancement of pro-apoptotic genes by p53. Furthermore, MCS induced apoptosis in MDA-MB-231 cells that had developed resistance to 5-FU and inhibited tumor growth in tumor-bearing mice with reduced 5-FU sensitivity. These findings suggest that an approach involving MCS could serve as a foundation for developing breast cancer treatment strategies to overcome p53 mutations.
Keywords: 5-fluorouracil; MDA-MB-231 cells; apoptosis; breast cancer; cell death; chemoresistance; flow cytometry; microcurrent; p53; reactive oxygen species (ROS).
Copyright © 2025 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures


Similar articles
-
Chemotherapy for advanced gastric cancer.Cochrane Database Syst Rev. 2017 Aug 29;8(8):CD004064. doi: 10.1002/14651858.CD004064.pub4. Cochrane Database Syst Rev. 2017. PMID: 28850174 Free PMC article.
-
Elucidating the Potential of E-cadherin Re-expression along with Trichostatin A and Zebularine in Enhancing Tumour Necrosis Factor-Related Apoptosis-Inducing Ligand (TRAIL)-Induced Apoptosis in Human Breast Adenocarcinoma Cells.Curr Cancer Drug Targets. 2025 Jun 20. doi: 10.2174/0115680096374361250610075556. Online ahead of print. Curr Cancer Drug Targets. 2025. PMID: 40574394
-
53BP1 loss induces chemoresistance of colorectal cancer cells to 5-fluorouracil by inhibiting the ATM-CHK2-P53 pathway.J Cancer Res Clin Oncol. 2017 Mar;143(3):419-431. doi: 10.1007/s00432-016-2302-5. Epub 2016 Nov 12. J Cancer Res Clin Oncol. 2017. PMID: 27838786 Free PMC article.
-
Thymidylate synthase inhibition induces p53-dependent and p53-independent apoptotic responses in human urinary bladder cancer cells.J Cancer Res Clin Oncol. 2011 Feb;137(2):359-74. doi: 10.1007/s00432-010-0891-y. Epub 2010 Apr 28. J Cancer Res Clin Oncol. 2011. PMID: 20425122 Free PMC article.
-
The use of irinotecan, oxaliplatin and raltitrexed for the treatment of advanced colorectal cancer: systematic review and economic evaluation.Health Technol Assess. 2008 May;12(15):iii-ix, xi-162. doi: 10.3310/hta12150. Health Technol Assess. 2008. PMID: 18462574
References
-
- Albain K.S., Barlow W.E., Shak S., Hortobagyi G.N., Livingston R.B., Yeh I.T., et al. Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: a retrospective analysis of a randomised trial. Lancet Oncol. 2010;11:55–65. - PMC - PubMed
-
- Martin M., Pienkowski T., Mackey J., Pawlicki M., Guastalla J.P., Weaver C., et al. Adjuvant docetaxel for node-positive breast cancer. N. Engl. J. Med. 2005;352:2302–2313. - PubMed
-
- Longley D.B., Harkin D.P., Johnston P.G. 5-fluorouracil: mechanisms of action and clinical strategies. Nat. Rev. Cancer. 2003;3:330–338. - PubMed
-
- Hafner A., Bulyk M.L., Jambhekar A., Lahav G. The multiple mechanisms that regulate p53 activity and cell fate. Nat. Rev. Mol. Cell Biol. 2019;20:199–210. - PubMed
-
- Ju J., Schmitz J.C., Song B., Kudo K., Chu E. Regulation of p53 expression in response to 5-fluorouracil in human cancer RKO cells. Clin. Cancer Res. 2007;13:4245–4251. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

