Phase II study of avelumab and trastuzumab with FOLFOX chemotherapy in previously untreated HER2-amplified metastatic gastroesophageal adenocarcinoma
- PMID: 40571483
- PMCID: PMC12269372
- DOI: 10.1093/oncolo/oyaf195
Phase II study of avelumab and trastuzumab with FOLFOX chemotherapy in previously untreated HER2-amplified metastatic gastroesophageal adenocarcinoma
Abstract
Background: Trastuzumab and multiagent chemotherapy have been the standard of care for the 20-30% of metastatic gastric and esophageal adenocarcinomas that overexpress HER2. Preclinical data show that trastuzumab requires a functional adaptive immune system for efficacy, suggesting synergy of trastuzumab combined with immune checkpoint inhibitors, further supported by current clinical studies.
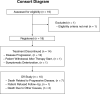
Methods: HCRN GI17-319 was a multicenter, single-arm, phase II clinical trial with a prespecified 6-subject safety run-in of the anti-PD-L1 antibody avelumab, combined with trastuzumab and mFOLFOX6, in previously untreated, metastatic, HER2-amplified gastric and esophageal adenocarcinomas. The primary endpoint was the best overall response within 24 weeks. Subjects received 9 cycles of induction avelumab, trastuzumab, and mFOLFOX6, followed by maintenance avelumab + trastuzumab. The study was initially designed as a Simon's 2-stage trial, but enrollment was stopped after the 18-subject first stage for reasons unrelated to safety or efficacy.
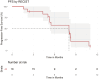
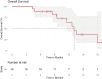
Results: A total of 18 subjects, including the 6-subject safety run-in, were enrolled 4/2019-8/2020. The 24-week response rate was 11/18 (61%; 95% CI: 39%-84%), and the confirmed overall response rate is 9/18 (50%). With a median follow-up of 14.6 months, the median PFS was 8.0 months (95% CI: 5.3-NA) and median OS was 13.1 months (95% CI: 11.5-NA). The regimen was well tolerated, without any new safety signals.
Conclusions: The combination of avelumab, trastuzumab, and FOLFOX chemotherapy demonstrated some activity, with a reasonable response rate and median PFS. These outcomes provide some support to other clinical trials of similar agents and support the future evaluation of adding avelumab in this setting. NCT03783936.
Keywords: HER2; esophageal cancer; gastric cancer; immunotherapy; trastuzumab.
© The Author(s) 2025. Published by Oxford University Press.
Conflict of interest statement
M.S. Lee: Consulting for Pfizer, Delcath, Janssen, BioNTech, G1 Therapeutics, Imvax, and Bayer. Research funding to the institution from EpimAb BioTherapeutics, Merck, Erasca, Boehringer Ingelheim, Arcus Biosciences, Repare Therapeutics, Trisalus Life Sciences, and Xilis. J. Chao: Research funding to institution from EMD Serono, Merck, Brooklyn Immunotherapeutics; Consulting for Eli Lilly, Merck, AstraZeneca, Foundation Medicine, Daiichi Sankyo, Macrogenics, Amgen, Ono Pharmaceuticals, Bristol-Myers Squibb, Astellas, Turning Point Therapeutics, Silverback Therapeutics, Novartis, Coherus Biosciences, Geneos, Roche; Speakers’ bureau for Merck and Bristol-Myers Squibb. P.M. Kasi: Scientific/Advisory Board: Eilcio, Founder: Percision BioSensors Inc. Consultancy/Advisory Board: Agenus, Astellas, AstraZeneca, Bayer, Beixon, BostonGene, Daiichi Sankyo, Delcath, Eli Lilly, Eisai, Elicio Therapeutics, Exact Sciences, Foundation Medicine, Guardant, Illumina, Ipsen, Merck, Natera, Neogenomics, QED, Regeneron, SAGA Diagnostics, Seagen, Servier, Taiho Oncology, Tempus, and Xilio Therapeutics. Grant Support: Agenus, Merck, Novartis. S. Mukherjee serves as a volunteer guidelines panel member at the National Comprehensive Cancer Network and American Society of Clinical Oncology, and research funding from the National Comprehensive Cancer Network and Ipsen Biopharmaceuticals/North American Neuroendocrine Tumor Society which were paid to the institute, and Mukherjee received a consulting fee from Merck, Eisai, and Beigene.
Figures

References
-
- Rha SY, Lee C-k, Kim HS, et al. A multi-institutional phase Ib/II trial of first-line triplet regimen (Pembrolizumab, Trastuzumab, Chemotherapy) for HER2-positive advanced gastric and gastroesophageal junction cancer (PANTHERA Trial): Molecular profiling and clinical update. J Clin Oncol. 2021;39:218-218. https://doi.org/ 10.1016/S1470-2045(23)00515-6 - DOI
-
- Janjigian YY, Maron SB, Chatila WK, et al. First-line pembrolizumab and trastuzumab in HER2-positive oesophageal, gastric, or gastro-oesophageal junction cancer: an open-label, single-arm, phase 2 trial. Lancet Oncol. 2020;21:821-831. https://doi.org/ 10.1016/S1470-2045(20)30169-8 - DOI - PMC - PubMed
-
- Janjigian YY, Kawazoe A, Yanez PE, et al. ; on behalf of the KEYNOTE-811 investigators. Pembrolizumab plus trastuzumab and chemotherapy for HER2+ metastatic gastric or gastroesophageal junction (G/GEJ) cancer: Initial findings of the global phase 3 KEYNOTE-811 study. J Clin Oncol. 2021;39:4013-4013. https://doi.org/ 10.1200/jco.2021.39.15_suppl.4013 - DOI
-
- Janjigian YY, Kawazoe A, Bai Y, et al. Pembrolizumab plus trastuzumab and chemotherapy for HER2-positive gastric or gastro-oesophageal junction adenocarcinoma: interim analyses from the phase 3 KEYNOTE-811 randomised placebo-controlled trial. Lancet. 2023;402:2197-2208. https://doi.org/ 10.1016/s0140-6736(23)02033-0 - DOI - PubMed
-
- Yoon HH, Bendell JC, Braiteh FS, et al. Ramucirumab combined with FOLFOX as front-line therapy for advanced esophageal, gastroesophageal junction, or gastric adenocarcinoma: a randomized, double-blind, multicenter Phase II trial. Ann Oncol. 2016;27:2196-2203. https://doi.org/ 10.1093/annonc/mdw423 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

