Integrin beta 1 facilitates non-enveloped hepatitis E virus cell entry through the recycling endosome
- PMID: 40571699
- PMCID: PMC12202797
- DOI: 10.1038/s41467-025-61071-y
Integrin beta 1 facilitates non-enveloped hepatitis E virus cell entry through the recycling endosome
Abstract
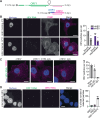
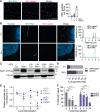
Hepatitis E virus (HEV) is a major cause of acute hepatitis and mainly transmitted faecal-orally. HEV particles present in faeces are naked (nHEV), whereas those found in the blood are quasi-enveloped (eHEV) with a cell-derived lipid membrane. Despite its global health impact, the cellular life cycle of HEV remains poorly understood, particularly regarding the mechanisms of viral entry into host cells. To address this knowledge gap, we develop a high content RNA-FISH-based imaging assay that allows for the investigation of the entry pathways of both naked and quasi-enveloped HEV particles. Surprisingly, we find that integrin α3, previously implicated in nHEV cell entry, is not expressed in the cell types that are most permissive for HEV infection. Instead, we identify integrin β1 (ITGB1) pairing with different α-integrins as the key player mediating nHEV cell entry. Our results indicate that the interaction of nHEV with ITGB1 facilitates entry through Rab11-positive recycling endosomes. In contrast, eHEV particles do not interact with ITGB1 and enter cells using a classical endocytic route via Rab5a-positive early endosomes. The entry of both types of HEV particles requires endosomal acidification and proteolytic cleavage by lysosomal cathepsins, which ultimately results in delivery of the HEV genome to the cytoplasm.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: All authors declare no conflict of interest.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

