Future perspectives of GMO detection in agriculture: strategies for electrochemical nucleic acid detection
- PMID: 40571859
- PMCID: PMC12202687
- DOI: 10.1007/s00604-025-07267-x
Future perspectives of GMO detection in agriculture: strategies for electrochemical nucleic acid detection
Abstract
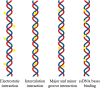
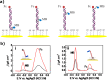
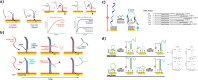
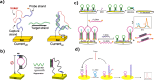
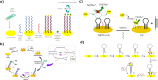
The uncontrolled distribution of genetically modified organisms (GMO)-based food and feed is an increasing global concern, primarily due to limited information about their potential harmful effects. The growing diversity and complexity of GMOs present significant challenges for their detection, traceability, and safety monitoring. Traditionally, GMOs are detected using molecular methods, among which PCR methods are the most explored and are considered the gold standard. However, isothermal nucleic acid amplification methods, though less explored, hold great potential, especially when integrated with biosensor platforms, enabling the development of highly efficient and versatile biosensing systems. This paper provides a comprehensive overview of the recent advances in biosensors utilizing methods of isothermal nucleic acid amplification, highlighting their current progress and future perspectives. We discuss molecular methods for GMO detection, focusing on reaction conditions, amplification efficiency, and compatibility with various detection modalities. Additionally, we investigate the integration of various nanomaterials into transducers, such as electrochemical platforms, together with the electrochemical techniques and detection mechanisms, aiming to outline their synergistic effects with molecular techniques to improve detection sensitivity and enable real-time monitoring. Furthermore, we discuss the applications of GMO biosensors across diverse fields, including food safety and environmental monitoring, while addressing existing challenges and potential strategies for improving the performance, robustness, and practicality of biosensing platforms. Overall, this review highlights the significant progress achieved in GMO biosensors and underscores their promising role in advancing diagnostic and monitoring capabilities.
Keywords: Electrochemical biosensors; Environmental monitoring; Field-effect transistor biosensors; Food safety; GMO; Nucleic acid amplification; Point-of-need; Transducers.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: The authors have no conflicts of interest to declare that are relevant to the content of this article. Clinical trial number: Not applicable.
Figures
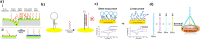





Similar articles
-
Current Progress on the Detection of Genetically Modified Organisms in Food: From Transgenic Towards Genome-Edited Crops.Compr Rev Food Sci Food Saf. 2025 Sep;24(5):e70243. doi: 10.1111/1541-4337.70243. Compr Rev Food Sci Food Saf. 2025. PMID: 40778611 Review.
-
CRISPR/Cas-powered nucleic acid amplification and amplification-free biosensors for public safety detection: Principles, advances and prospects.Biotechnol Adv. 2025 Oct;83:108609. doi: 10.1016/j.biotechadv.2025.108609. Epub 2025 May 21. Biotechnol Adv. 2025. PMID: 40409480 Review.
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
-
Innovations in aptamer-based biosensors for detection of pathogenic bacteria: Recent advances and perspective.Talanta. 2025 Dec 1;295:128330. doi: 10.1016/j.talanta.2025.128330. Epub 2025 May 14. Talanta. 2025. PMID: 40388877 Review.
-
Research Progress on Aptamer Electrochemical Biosensors Based on Signal Amplification Strategy.Sensors (Basel). 2025 Jul 12;25(14):4367. doi: 10.3390/s25144367. Sensors (Basel). 2025. PMID: 40732494 Free PMC article. Review.
References
-
- Peter R, Mojca J, Primož P (2011) Genetically modified organisms (GMOs). In: Encyclopedia of Environmental Health. pp. 879–888. Elsevier
-
- Uslu T (2021) Advantages, risks and legal perspectives of GMOs in 2020s. Plant Biotechnol Rep 15:741–751. 10.1007/s11816-021-00714-0
-
- Zilberman D, Holland TG, Trilnick I (2018) Agricultural GMOs—what we know and where scientists disagree. Sustainability 10:1514. 10.3390/su10051514
Publication types
MeSH terms
Substances
Grants and funding
- 6710/Science Fund of the Republic of Serbia
- 6710/Science Fund of the Republic of Serbia
- 6710/Science Fund of the Republic of Serbia
- 6710/Science Fund of the Republic of Serbia
- 6710/Science Fund of the Republic of Serbia
- 6710/Science Fund of the Republic of Serbia
- 6710/Science Fund of the Republic of Serbia
- 6710/Science Fund of the Republic of Serbia
- 6710/Science Fund of the Republic of Serbia
- 451-03-136/2025-03/200358/Ministarstvo nauke, tehnološkog razvoja i inovacija, Republika Srbija
- 451-03-136/2025-03/200358/Ministarstvo nauke, tehnološkog razvoja i inovacija, Republika Srbija
- 451-03-136/2025-03/200358/Ministarstvo nauke, tehnološkog razvoja i inovacija, Republika Srbija
- 451-03-136/2025-03/200358/Ministarstvo nauke, tehnološkog razvoja i inovacija, Republika Srbija
- 451-03-136/2025-03/200358/Ministarstvo nauke, tehnološkog razvoja i inovacija, Republika Srbija
- 451-03-136/2025-03/200358/Ministarstvo nauke, tehnološkog razvoja i inovacija, Republika Srbija
- 451-03-136/2025-03/200358/Ministarstvo nauke, tehnološkog razvoja i inovacija, Republika Srbija
- 451-03-136/2025-03/200358/Ministarstvo nauke, tehnološkog razvoja i inovacija, Republika Srbija
LinkOut - more resources
Full Text Sources
