Traditional Chinese Medicine, Ziyin-Mingmu Decoction, Regulates Cholesterol Metabolism, Oxidative Stress, Inflammation and Gut Microbiota in Age-related Macular Degeneration Models
- PMID: 40571871
- PMCID: PMC12304070
- DOI: 10.1007/s11095-025-03887-3
Traditional Chinese Medicine, Ziyin-Mingmu Decoction, Regulates Cholesterol Metabolism, Oxidative Stress, Inflammation and Gut Microbiota in Age-related Macular Degeneration Models
Abstract
Background: Age-related macular degeneration (AMD) is the commonest cause of retinal disorders in the aged population. Ziyin-Mingmu decoction (ZD) has been widely used to treat AMD patients over thousands of years, however the underlying functional mechanisms of ZD are largely elusive. In this study, we aim to elucidate the therapeutic mechanisms of ZD in AMD models.
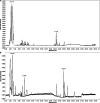
Methods: An in vivo AMD mouse model and an in vitro AMD model were established. Cholesterol level in mouse tissues was measured. Expression of antioxidant genes and proinflammatory cytokines in mouse tissues and in human retinal pigment epithelial (RPE) cells were detected using biochemical approaches. Gut microbiota community and functional pathways were analysed using bioinformatics approach. Compounds in ZD were identified using HPLC/MS.
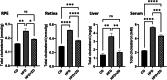
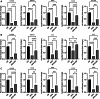
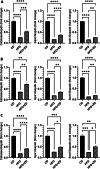
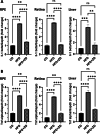
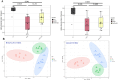
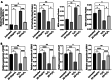
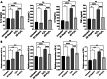
Results: High fat diet (HFD)-fed mice had significantly higher levels of cholesterol in the retina, RPE, liver and serum, and markedly decreased expression of cholesterol metabolism-associated genes in those tissues, compared to mice fed with normal diet. Similarly, expression of antioxidant and inflammation genes was dysregulated in HFD-fed mouse tissues. ZD treatment reversed these HFD-induced pathological effects. HFD also altered the composition of cecum bacterial communities and associated metabolic pathways, which returned to control levels by ZD. In vitro assays showed that H2O2 significantly increased oxidative stress and enhanced expression of proinflammatory cytokines. Co-treatment with ZD significantly counteracted these changes. HPLC/MS identified 105 compound in water extracted ZD and most are polyphenols.
Conclusion: Our data suggests that protection of ZD against AMD is possibly through mitigating cholesterol level, oxidative stress and inflammation, and modulating gut microbiota by polyphenols.
Keywords: age-related macular degeneration; cholesterol; gut microbiota; inflammation; oxidative stress; ziyin-mingmu decoction.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: The authors have no conflicts of interest to declare. Institutional Review Board Statement: The animal work of this study was approved by the Animal Ethics and Welfare Committee, Hunan University of Chinese Medicine (SYXK (Xiang) 2019- 0009, approved date 10 January 2019).
Figures



Similar articles
-
PGC-1α repression dysregulates lipid metabolism and induces lipid droplet accumulation in retinal pigment epithelium.Cell Death Dis. 2024 Jun 1;15(6):385. doi: 10.1038/s41419-024-06762-y. Cell Death Dis. 2024. PMID: 38824126 Free PMC article.
-
Chinese medicine, Qijudihuang pill, mediates cholesterol metabolism and regulates gut microbiota in high-fat diet-fed mice, implications for age-related macular degeneration.Front Immunol. 2023 Oct 12;14:1274401. doi: 10.3389/fimmu.2023.1274401. eCollection 2023. Front Immunol. 2023. PMID: 37901244 Free PMC article.
-
Study on the modulation of kidney and liver function of rats with diabetic nephropathy by Huidouba through metabolomics.J Ethnopharmacol. 2025 Jul 24;351:120136. doi: 10.1016/j.jep.2025.120136. Epub 2025 Jun 11. J Ethnopharmacol. 2025. PMID: 40513925
-
Artificial intelligence for diagnosing exudative age-related macular degeneration.Cochrane Database Syst Rev. 2024 Oct 17;10(10):CD015522. doi: 10.1002/14651858.CD015522.pub2. Cochrane Database Syst Rev. 2024. PMID: 39417312
-
Anti-vascular endothelial growth factor biosimilars for neovascular age-related macular degeneration.Cochrane Database Syst Rev. 2024 Jun 3;6(6):CD015804. doi: 10.1002/14651858.CD015804.pub2. Cochrane Database Syst Rev. 2024. PMID: 38829176 Free PMC article.
References
-
- Wong WL, Su X, Li X, Cheung CMG, Klein R, Cheng CY, Wong TY. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health. 2014;2(2):e106–16. - PubMed
-
- Guymer RH, Campbell TG. Age-related macular degeneration. The Lancet. 2023;401(10386):1459–72. - PubMed
-
- Rudnicka AR, Jarrar Z, Wormald R, Cook DG, Fletcher A, Owen CG. Age and gender variations in age-related macular degeneration prevalence in populations of European ancestry: a meta-analysis. Ophthalmol. 2012;119(3):571–80. - PubMed
-
- Thornton J, Edwards R, Mitchell P, Harrison RA, Buchan I, Kelly SP. Smoking and age-related macular degeneration: a review of association. Eye. 2005;19(9):935–44. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

