Chlorophyllin-Mediated Photodynamic Inactivation: Dosage and Time Dependency in the Inhibition of Bacillus subtilis
- PMID: 40572076
- PMCID: PMC12195115
- DOI: 10.3390/microorganisms13061189
Chlorophyllin-Mediated Photodynamic Inactivation: Dosage and Time Dependency in the Inhibition of Bacillus subtilis
Abstract
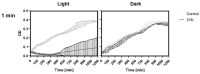
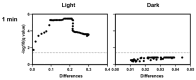
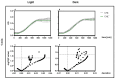
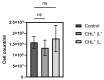
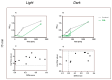
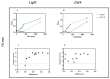
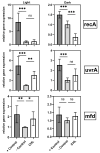
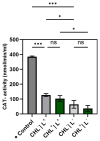
Photodynamic inactivation of bacteria offers a promising alternative to counteract the trend towards the development of resistance, which, if left uncontrolled, will lead to the death of 10 million people per year by 2050. Its advantage over antibiotics is the site-specific mode of action due to the photosensitizer (PS) and the low risk of developing resistance. This is primarily prevented by the damage of the bacteria, which also destroy internal structures such as nucleic acid, proteins, and lipids. A promising and still little-researched PS is chlorophyllin (CHL), a chlorophyll derivative. This study investigated its mode of action on Bacillus subtilis growth using optical density (OD) measurements. It was shown that the PS is highly effective even at low concentrations and short irradiation durations. Here, 1 mg/L and an irradiation duration of 1 min were sufficient to inhibit the growth of the Gram-positive bacterium Bacillus subtilis for several hours.
Keywords: antibiotic alternatives; chlorophyllin; photodynamic inactivation (PDI); photodynamic therapy (PDT); photosensitizer.
Conflict of interest statement
Author Michael Lebert is engaged with the company Space Biology Unlimited, SAS. There is no conflict of interest regarding this MS. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
-
Home treatment for mental health problems: a systematic review.Health Technol Assess. 2001;5(15):1-139. doi: 10.3310/hta5150. Health Technol Assess. 2001. PMID: 11532236
-
The safety and efficiency of photodynamic therapy for the treatment of osteosarcoma: A systematic review of in vitro experiment and animal model reports.Photodiagnosis Photodyn Ther. 2022 Dec;40:103093. doi: 10.1016/j.pdpdt.2022.103093. Epub 2022 Aug 27. Photodiagnosis Photodyn Ther. 2022. PMID: 36031143
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer.Health Technol Assess. 2001;5(32):1-195. doi: 10.3310/hta5320. Health Technol Assess. 2001. PMID: 12065068
-
Nivolumab for adults with Hodgkin's lymphoma (a rapid review using the software RobotReviewer).Cochrane Database Syst Rev. 2018 Jul 12;7(7):CD012556. doi: 10.1002/14651858.CD012556.pub2. Cochrane Database Syst Rev. 2018. PMID: 30001476 Free PMC article.
References
-
- O’Neill J. Review on Antimicrobial Resistance. Antimicrobial Resistance: Tackling a Crisis for the Health and Wealth of Nations. Volume 2014. Review on Antimicrobial Resistance; London, UK: 2014. pp. 1–16.
Grants and funding
LinkOut - more resources
Full Text Sources

