Porcine Teschovirus 2 3Cpro Evades Host Antiviral Innate Immunity by Inhibiting the IFN-β Signaling Pathway
- PMID: 40572097
- PMCID: PMC12195036
- DOI: 10.3390/microorganisms13061209
Porcine Teschovirus 2 3Cpro Evades Host Antiviral Innate Immunity by Inhibiting the IFN-β Signaling Pathway
Abstract
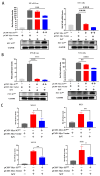
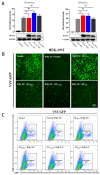
Porcine teschovirus (PTV) circulates in pig populations, causing clinical diseases such as poliomyelitis, reproductive disorders, and pneumonia. However, the molecular mechanisms underlying the pathogenesis of PTV infection have not been fully elucidated. Here, we found that PTV infection does not activate the promoters of NF-κB or IFN-β. The expression of PTV 3Cpro inhibits the promoter activity of NF-κB and IFN-β stimulated by SeV and inhibits the downstream transcription of NF-κB and IFN-β by blocking the phosphorylation and nuclear translocation of NF-κB. Coimmunoprecipiation (co-IP) experiments demonstrated that 3Cpro and NF-κB interact. The degradation of NF-κB was unaffected by inhibitors targeting lysosomes (NH4Cl), proteasomes (MG132), or caspases (Z-VAD-FMK). The protease activity of 3Cpro, which relies on its catalytic active site, is vital for NF-κB cleavage and degradation. Loss of proteolytic activity in mutants abolished NF-κB degradation, impairing the ability of 3Cpro to suppress SeV-induced innate immunity and restore VSV-GFP replication, thereby underscoring its critical role in immune evasion by targeting NF-κB. This study reveals novel mechanisms underlying PTV-mediated suppression of host innate immunity.
Keywords: 3Cpro; NF-κB; inhibitor; porcine teschovirus; protease activity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Trefny L. Massive illness of swine in Teschen area. Zveroleki Obz. 1930;23:235–236.
Grants and funding
LinkOut - more resources
Full Text Sources

