A Comprehensive Safety Assessment of Ralstonia eutropha H16 for Food Applications: Integrating Genomic, Phenotypic, and Toxicological Analyzes
- PMID: 40572211
- PMCID: PMC12195361
- DOI: 10.3390/microorganisms13061323
A Comprehensive Safety Assessment of Ralstonia eutropha H16 for Food Applications: Integrating Genomic, Phenotypic, and Toxicological Analyzes
Abstract
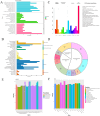
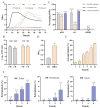
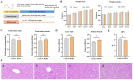
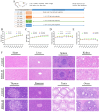
Ralstonia eutropha H16, a metabolically versatile bacterium, has gained prominence as a microbial platform for sustainable bioproduction. While its capabilities in synthesizing single-cell proteins and biodegradable materials are well documented, comprehensive strain-level safety evaluations remain insufficient for food-grade applications. This study systematically assessed the safety of R. eutropha H16 through genomic, phenotypic, and toxicological analyzes. Genomic analyzes revealed the absence or minimal presence of virulence factors and antibiotic resistance genes, aligning with microbiological safety standards. Phenotypic investigations demonstrated a limited gastric fluid tolerance (pH 2.5, survival rate 25.70% after 3 h) and intestinal fluid persistence (pH 8, 44.67% viability after 3 h), coupled with an exceptional bile salt tolerance (0.2% w/v). Antioxidant assays confirmed the fermentation broth specifically scavenges DPPH free radicals (14.60 ± 1.24 μg Trolox/mL), whereas bacterial suspensions and cell-free supernatants exhibited a strong hydroxyl radical scavenging (>90 U/mL) and superoxide anion inhibition (>100 U/L). Acute toxicity testing indicated no mortality or histopathological abnormalities, with an LD50 value exceeding 1 × 10¹¹ CFU/kg. Subacute toxicity studies (28-day, 1 × 108-1 × 1010 CFU/kg) revealed no significant effects on growth, hematology, or organ function. Minor alterations in serum biochemistry might be attributed to physiological adaptation. Subacute exposure induced transient serum ALT fluctuations without hepatorenal dysfunction, while maintaining hematological parameters within physiological ranges. Collectively, these results substantiate the safety of R. eutropha H16 for food-related applications while underscoring the necessity of strain-specific risk assessments for industrial microbial platforms.
Keywords: Ralstonia eutropha H16; acute and subacute toxicity; phenotypic analysis; safety assessment.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures
Similar articles
-
Drugs for preventing postoperative nausea and vomiting in adults after general anaesthesia: a network meta-analysis.Cochrane Database Syst Rev. 2020 Oct 19;10(10):CD012859. doi: 10.1002/14651858.CD012859.pub2. Cochrane Database Syst Rev. 2020. PMID: 33075160 Free PMC article.
-
What is the value of routinely testing full blood count, electrolytes and urea, and pulmonary function tests before elective surgery in patients with no apparent clinical indication and in subgroups of patients with common comorbidities: a systematic review of the clinical and cost-effective literature.Health Technol Assess. 2012 Dec;16(50):i-xvi, 1-159. doi: 10.3310/hta16500. Health Technol Assess. 2012. PMID: 23302507 Free PMC article.
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
-
Effects of a gluten-reduced or gluten-free diet for the primary prevention of cardiovascular disease.Cochrane Database Syst Rev. 2022 Feb 24;2(2):CD013556. doi: 10.1002/14651858.CD013556.pub2. Cochrane Database Syst Rev. 2022. PMID: 35199850 Free PMC article.
-
A systematic review and economic evaluation of epoetin alpha, epoetin beta and darbepoetin alpha in anaemia associated with cancer, especially that attributable to cancer treatment.Health Technol Assess. 2007 Apr;11(13):1-202, iii-iv. doi: 10.3310/hta11130. Health Technol Assess. 2007. PMID: 17408534
References
-
- Jiang Y., Yang X., Zeng D., Su Y., Zhang Y. Microbial conversion of syngas to single cell protein: The role of carbon monoxide. Chem. Eng. J. 2022;450:138041. doi: 10.1016/j.cej.2022.138041. - DOI
-
- Lee Y.J., Moon B.C., Lee D.K., Ahn J.H., Gong G., Um Y., Lee S.-M., Kim K.H., Ko J.K. Sustainable production of microbial protein from carbon dioxide in the integrated bioelectrochemical system using recycled nitrogen sources. Water Res. 2025;268:122576. doi: 10.1016/j.watres.2024.122576. - DOI - PubMed
Grants and funding
- XDC 0110300/Strategic Priority Research Program of the Chinese Academy of Sciences
- E2M9560201/Major Project of Haihe Laboratory of Synthetic Biology
- TSBICIP-CXRC-008/Tianjin Synthetic Biotechnology Innovation Capacity Improvement Projects
- 32301210 & 31200035/National Natural Science Foundation of China
- No. 252300421412/Henan Provincial Natural Science Foundation
LinkOut - more resources
Full Text Sources
Research Materials

