Identification, Comparison, and Profiling of Selected Diarrhoeagenic Pathogens from Diverse Water Sources and Human and Animal Faeces Using Whole-Genome Sequencing
- PMID: 40572261
- PMCID: PMC12196432
- DOI: 10.3390/microorganisms13061373
Identification, Comparison, and Profiling of Selected Diarrhoeagenic Pathogens from Diverse Water Sources and Human and Animal Faeces Using Whole-Genome Sequencing
Abstract
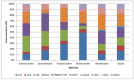
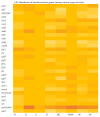
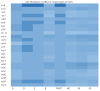
Consumption of contaminated drinking water is known to cause waterborne diseases such as diarrhoea, dysentery, typhoid, and hepatitis. This study applied whole-genome sequencing (WGS) to detect, identify, compare, and profile diarrhoeagenic pathogens (Vibrio cholerae, Shiga toxin-producing Escherichia coli, and Escherichia coli O157:H7) from 3168 water samples and 135 faecal samples (human and animal). Culture-based methods, MALDI-TOF mass spectrometry, and PCR were employed prior to WGS for identification of pathogens. Culture-based results revealed high presumptive prevalence of STEC (40.2%), V. cholerae (37.1%), and E. coli O157:H7 (22.7%). The MALDI-TOF confirmed 555 isolates with V. cholerae identified as Vibrio albensis. Shiga toxin-producing Escherichia coli (STEC) was more prevalent in wastewater (60%), treated water (54.1%), and groundwater (36.8%). PCR detected 46.4% of virulence genes from the water isolates and 66% of virulence genes from the STEC stool isolates. WGS also revealed STEC (92.9%) as the most prevalent species and found common virulence (e.g., hcp1/tssD1 and hlyE) and resistance (e.g., acrA and baeR) genes in all three types of samples. Five resistance and thirteen virulence genes overlapped among treated water and stool isolates. These findings highlight the diarrhoeagenic pathogens' public health risk in water sources and underscore the need for better water quality monitoring and treatment standards.
Keywords: MALDI-TOF MS; conventional PCR; rural communities; water sources; whole-genome sequencing (WGS).
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Molecular characterization of enteropathogenic Escherichia coli isolated from patients with gastroenteritis in a tertiary referral hospital of northeast India.Indian J Med Microbiol. 2024 Jan-Feb;47:100535. doi: 10.1016/j.ijmmb.2024.100535. Epub 2024 Feb 15. Indian J Med Microbiol. 2024. PMID: 38350526
-
The Study of Escherichia coli as Antimicrobial-Resistant Sentinel Microorganism Isolated in the Farms of Three Districts of Ankara by MALDI-TOF MS and Genomic Analysis.Vet Med Sci. 2025 May;11(3):e70209. doi: 10.1002/vms3.70209. Vet Med Sci. 2025. PMID: 40173253 Free PMC article.
-
Occurrence and Genotypic Characterization of Selected Multidrug-resistant ESKAPE-E Pathogens Isolated from Integrated Smallholder Fresh Produce Farms.J Food Prot. 2025 Jun 23;88(7):100543. doi: 10.1016/j.jfp.2025.100543. Epub 2025 May 16. J Food Prot. 2025. PMID: 40383269 Free PMC article.
-
Interventions to improve sanitation for preventing diarrhoea.Cochrane Database Syst Rev. 2023 Jan 25;1(1):CD013328. doi: 10.1002/14651858.CD013328.pub2. Cochrane Database Syst Rev. 2023. PMID: 36697370 Free PMC article.
-
Diagnostic test accuracy and cost-effectiveness of tests for codeletion of chromosomal arms 1p and 19q in people with glioma.Cochrane Database Syst Rev. 2022 Mar 2;3(3):CD013387. doi: 10.1002/14651858.CD013387.pub2. Cochrane Database Syst Rev. 2022. PMID: 35233774 Free PMC article.
References
-
- WHO . Guidelines for Drinking Water Quality: Fourth Edition, Incoporating the First and Second Addenda. WHO; Geneva, Switzerland: 2022. - PubMed
-
- Edokpayi J.N., Rogawski E.T., Kahler D.M., Hill C.L., Reynolds C., Nyathi E., Smith J.A., Odiyo J.O., Samie A., Bessong P., et al. Challenges to sustainable safe drinking water: A case study of water quality and use across seasons in rural communities in Limpopo Province, South Africa. Water. 2018;10:159. doi: 10.3390/w10020159. - DOI - PMC - PubMed
-
- Amatobi D.A., Agunwamba J.C. Improved quantitative microbial risk assessment (QMRA) for drinking water sources in developing countries. Appl. Water Sci. 2022;12:49. doi: 10.1007/s13201-022-01569-8. - DOI
-
- Karama M., Mainga A.O., Cenci-Goga B.T., Malahlela M., El-Ashram S., Kalake A. Molecular profiling and antimicrobial resistance of Shiga toxin-producing Escherichia coli O26, O45, O103, O121, O145 and O157 isolates from cattle on cow-calf operations in South Africa. Sci. Rep. 2019;9:11930. doi: 10.1038/s41598-019-47948-1. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

