Molecular Characterization of a Transcriptional Regulator GntR for Gluconate Metabolism in Industrial 2-Ketogluconate Producer Pseudomonas plecoglossicida JUIM01
- PMID: 40572283
- PMCID: PMC12195362
- DOI: 10.3390/microorganisms13061395
Molecular Characterization of a Transcriptional Regulator GntR for Gluconate Metabolism in Industrial 2-Ketogluconate Producer Pseudomonas plecoglossicida JUIM01
Abstract
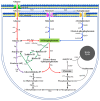
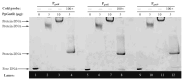
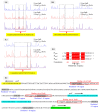
The GntR is a transcriptional regulator generally known as a gluconate-operon repressor to specifically regulate the transportation and phosphorylation of gluconate. In the present study we report the cloning of the GntR-encoding gene of the industrial 2-ketogluconate (2KGA)-producer Pseudomonas plecoglossicida JUIM01, which is involved in the regulation of gluconate metabolism, along with the identification of some of its target genes and its operator sequence. GntR is a 36.36-kDa cytoplasmic and hydrophobic DNA-binding transcriptional regulator belonging to the LacI family. The knockout of gntR resulted in the significant upregulation of the transcription of the gluconate kinase gene gntK and, to a lesser extent, the permease gene gntP, as well as downregulation of genes involved in glucose uptake (oprB-1, gltB, gltF, gltG, and gltK) and those involved in 2-ketogluconate (2KGA) transport (kguT) and catabolism (kguE, kguK, and kguD). These results indicated that GntR positively regulated glucose and 2KGA transport and catabolism, while negatively affecting GntP-mediated gluconate uptake and gluconate phosphorylation by GntK. Electrophoretic mobility shift assay (EMSA) and DNase I footprinting analyses confirmed that GntR interacted with operator sequences in the divergent promoter regions of gntK and gntP, as well as in the gntR promoter region. A putative operator sequence (consensus 5'-AG-N2-AGCGCT-N-TCT-3') was identified. These data suggest that GntR positively regulates genes involved in glucose uptake/transport and 2KGA transport/catabolism, while repressing its own expression as well as that of genes involved in gluconate transport/catabolism. These findings not only elucidate the regulation of GntR and its target genes in P. plecoglossicida, but also provide valuable insights for optimizing industrial 2KGA production.
Keywords: 2-ketogluconate (2KGA); LacI family transcriptional regulator GntR; Pseudomonas plecoglossicida; gluconate metabolism; specific binding sites.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Characterization and Transcriptional Regulation of the 2-Ketogluconate Utilization Operon in Pseudomonas plecoglossicida.Microorganisms. 2024 Dec 8;12(12):2530. doi: 10.3390/microorganisms12122530. Microorganisms. 2024. PMID: 39770733 Free PMC article.
-
The Role of kguT Gene in 2-Ketogluconate-Producing Pseudomonas plecoglossicida JUIM01.Appl Biochem Biotechnol. 2019 Mar;187(3):965-974. doi: 10.1007/s12010-018-2843-y. Epub 2018 Aug 14. Appl Biochem Biotechnol. 2019. PMID: 30109560
-
A 2-ketogluconate kinase KguK in Pseudomonas plecoglossicida JUIM01: Enzymatic characterization and its role in 2-keto-d-gluconic acid metabolism.Int J Biol Macromol. 2020 Dec 15;165(Pt B):2640-2648. doi: 10.1016/j.ijbiomac.2020.10.169. Epub 2020 Oct 26. Int J Biol Macromol. 2020. PMID: 33122059
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
-
Psychological interventions for adults who have sexually offended or are at risk of offending.Cochrane Database Syst Rev. 2012 Dec 12;12(12):CD007507. doi: 10.1002/14651858.CD007507.pub2. Cochrane Database Syst Rev. 2012. PMID: 23235646 Free PMC article.
References
-
- Grüning N.M., Ralser M. Glycolysis: How a 300 year long research journey that started with the desire to improve alcoholic beverages kept revolutionizing biochemistry. Curr. Opin. Syst. Biol. 2021;28:100380. doi: 10.1016/j.coisb.2021.100380. - DOI
-
- Nikel P.I., Chavarría M., Fuhrer T., Sauer U., de Lorenzo V. Pseudomonas putida KT2440 strain metabolizes glucose through a cycle formed by enzymes of the Entner-Doudoroff, Embden-Meyerhof-Parnas, and pentose phosphate pathways. J. Biol. Chem. 2015;290:25920–25932. doi: 10.1074/jbc.M115.687749. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

