Impact of c-di-AMP Accumulation, L-cysteine, and Oxygen on Catalase Activity and Oxidative Stress Resistance of Listeria monocytogenes 10403S
- PMID: 40572288
- PMCID: PMC12196104
- DOI: 10.3390/microorganisms13061400
Impact of c-di-AMP Accumulation, L-cysteine, and Oxygen on Catalase Activity and Oxidative Stress Resistance of Listeria monocytogenes 10403S
Abstract
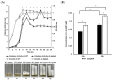
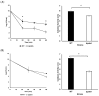
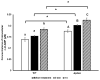
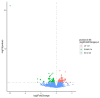
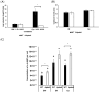
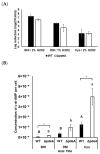
Listeria monocytogenes is a foodborne pathogen frequently exposed to oxidative stress in diverse environmental conditions. Cyclic di-AMP (c-di-AMP) is a second messenger that plays a key role in stress resistance. This study investigates the role of pdeA (degrades c-di-AMP) and how c-di-AMP accumulation affects catalase activity and oxidative stress response and gene expression. Survival and catalase activity assays were conducted under oxidative stress, and c-di-AMP levels were quantified in L. monocytogenes 10403S under aerobic, anaerobic, and L-cysteine-supplemented conditions. ΔpdeA, which accumulates c-di-AMP, exhibited greater sensitivity to oxidative stress (4.6 log reduction for the wild type (WT) vs 7.34 log reduction for ΔpdeA at 10 h) and lower catalase activity than the WT in the early stationary phase. However, in the late stationary phase, while the catalase activity levels of ΔpdeA remained stable (~6.33 cm foam height), it became resistant to oxidative stress (5.85 log reduction). These findings indicate that pdeA contributes to catalase activity in L. monocytogenes. Transcriptomic analysis revealed differential expression of pathways mainly including pentose phosphate pathway, carbon metabolism, O-antigen nucleotide sugar biosynthesis and ABC transporters in ΔpdeA compared to WT. Our transcriptomic data provided promising insights into the molecular mechanisms underlying c-di-AMP regulation, which may enhance stress resistance. Moreover, oxidative stress led to increased intracellular c-di-AMP levels. Under L-cysteine supplementation, catalase activity levels in WT were similar to ΔpdeA (~1.86 cm foam height for both), but the latter showed enhanced oxidative stress resistance and c-di-AMP levels. Anaerobic conditions also elevated c-di-AMP levels in WT and ΔpdeA but resulted in greater oxidative stress sensitivity. Understanding these regulatory mechanisms provides valuable insights into oxidative stress resistance, with potential implications for food safety and pathogen control.
Keywords: Listeria monocytogenes; c-di-AMP accumulation; catalase activity; cysteine; oxidative stress resistance; phosphodiesterase; second messenger.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Acid or salt adaptation of Listeria monocytogenes 10403S grown until exponential phase aerobically, enhances sensitivity to oxidative stress.J Appl Microbiol. 2025 Jul 1;136(7):lxaf173. doi: 10.1093/jambio/lxaf173. J Appl Microbiol. 2025. PMID: 40657895
-
Impact of pre-growth and storage conditions on the survival of Listeria monocytogenes in acidic baby fruit purees: Implications for food safety and consumer practices.Food Res Int. 2025 Oct;217:116861. doi: 10.1016/j.foodres.2025.116861. Epub 2025 Jun 11. Food Res Int. 2025. PMID: 40597558
-
Beyond antimicrobial resistance: MATE-type efflux pump FepA contributes to flagellum formation and virulence in Listeria monocytogenes.Appl Environ Microbiol. 2025 Jul 23;91(7):e0046225. doi: 10.1128/aem.00462-25. Epub 2025 Jun 10. Appl Environ Microbiol. 2025. PMID: 40492698 Free PMC article.
-
Home treatment for mental health problems: a systematic review.Health Technol Assess. 2001;5(15):1-139. doi: 10.3310/hta5150. Health Technol Assess. 2001. PMID: 11532236
-
The effectiveness and cost-effectiveness of carmustine implants and temozolomide for the treatment of newly diagnosed high-grade glioma: a systematic review and economic evaluation.Health Technol Assess. 2007 Nov;11(45):iii-iv, ix-221. doi: 10.3310/hta11450. Health Technol Assess. 2007. PMID: 17999840
References
Grants and funding
LinkOut - more resources
Full Text Sources

