Adaptive Laboratory Evolution of a Microbial Consortium Enhancing Non-Protein Nitrogen Assimilation for Feed Protein Production
- PMID: 40572304
- PMCID: PMC12195778
- DOI: 10.3390/microorganisms13061416
Adaptive Laboratory Evolution of a Microbial Consortium Enhancing Non-Protein Nitrogen Assimilation for Feed Protein Production
Abstract
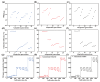
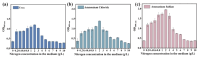
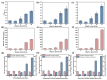
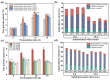
The increasing global demand for protein underscores the necessity for sustainable alternatives to soybean-based animal feed, which poses a challenge to human food security. Thus, the search for sustainable, alternative protein sources is transforming the feed industry in its effort to sustainable operations. In this study, a microbial consortium was subjected to adaptive laboratory evolution using non-protein nitrogen (NPN) and wheat straw as the sole carbon source. The evolved microbial consortium was subsequently utilized to perform solid-state fermentation on wheat straw and NPN to produce feed protein. After 20 generations, the microbial consortium demonstrated tolerance to 5 g/L NPN, including ammonium sulfate, ammonium chloride, and urea, which represents a fivefold increase compared to the original microbial consortium. Among the three NPNs tested, the evolved microbial consortium exhibited optimal growth performance with ammonium sulfate. Subsequently, the evolved microbial consortium was employed for the solid-state fermentation (SSF) of wheat straw, and the fermentation conditions were optimized. It was found that the true protein content of wheat straw could be increased from 2.74% to 10.42% under specific conditions: ammoniated wheat straw (15% w/w), non-sterilization of the substrate, an inoculation amount of 15% (v/w), nitrogen addition amount of 0.5% (w/w), an initial moisture content of 70%, a fermentation temperature of 30 °C, and a fermentation duration of 10 days. Finally, the SSF process for wheat straw was successfully scaled up from 0.04 to 2.5 kg, resulting in an increased true protein content of 9.84%. This study provides a promising approach for the production of feed protein from straw and NPN through microbial fermentation, addressing protein resource shortages in animal feed and improving the value of waste straw.
Keywords: adaptive laboratory evolution; microbial consortium; non-protein nitrogen assimilation; solid-state fermentation; wheat straw.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Zhang Z., Chen X., Gao L. New strategy for the biosynthesis of alternative feed protein: Single-cell protein production from straw-based biomass. GCB Bioenergy. 2024;16:e13120. doi: 10.1111/gcbb.13120. - DOI
-
- Wang R., Lin F., Feng K. Soybean overweight shock (SOS): The impact of trade liberalization in China on overweight prevalence. China Econ. Rev. 2024;87:102224. doi: 10.1016/j.chieco.2024.102224. - DOI
-
- Kong S., Wang S., He Y., Wang N., Wang Z., Weng L., Liu D., Zhao X., Chen J., Xu J., et al. Three-stage solid-state fermentation technology for Distillers’ Grain feed protein based on different microorganisms considering oxygen requirements. Fermentation. 2024;10:550. doi: 10.3390/fermentation10110550. - DOI
Grants and funding
- 22308338/the National Natural Science Foundation of China
- 52200178/the National Natural Science Foundation of China
- CX(24)1009/Jiangsu Agriculture Science and Technology Innovation Fund
- 21XTCX21001/Key Program for Collaborative and Innovation of Nanyang
- 252102110055/Henan Provincial Key Technology Research and Development Program
LinkOut - more resources
Full Text Sources

