Development of a Fluorescent Rapid Test Sensing System for Influenza Virus
- PMID: 40572355
- PMCID: PMC12195583
- DOI: 10.3390/mi16060635
Development of a Fluorescent Rapid Test Sensing System for Influenza Virus
Abstract
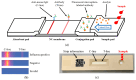
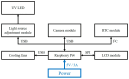
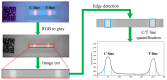
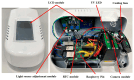
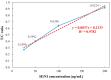
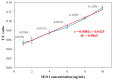
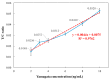
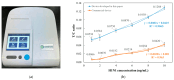
This paper presents a sensitive and stable fluorescence rapid test sensing system for the quantitative analysis of influenza rapid test results, integrating a detection reader to minimize errors from conventional visual interpretation. The hardware includes a control board, touchscreen, camera module, UV LED illumination, and a dark chamber, while the software handles camera and light source control, as well as image processing. Validation shows strong linearity, high precision, and reproducibility. For influenza A (H1N1), the system achieved a coefficient of determination (R2) of 0.9782 (25-200 ng/mL) and 0.9865 (1-10 ng/mL); for influenza B (Yamagata), the coefficient of determination (R2) was 0.9762 (2-10 ng/mL). The coefficient of variation ranged from 1-5% for influenza A and 4-9% for influenza B. Detection limits were 4 ng/mL for influenza A and 6 ng/mL for influenza B. These results confirm the system's capability for accurate quantitative analysis while reducing reliance on subjective interpretation. Its compact, portable design supports on-site rapid testing and allows for potential expansion to detect other targets, such as COVID-19, RSV, and myocardial enzymes. The system's scalability makes it a promising tool for clinical diagnostics, point-of-care testing (POCT), and infectious disease monitoring.
Keywords: fluorescence immunoassay (FIA); image processing; influenza detection; optical detection; rapid test detection; sensing system.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Rapid, point-of-care antigen tests for diagnosis of SARS-CoV-2 infection.Cochrane Database Syst Rev. 2022 Jul 22;7(7):CD013705. doi: 10.1002/14651858.CD013705.pub3. Cochrane Database Syst Rev. 2022. PMID: 35866452 Free PMC article.
-
Eliciting adverse effects data from participants in clinical trials.Cochrane Database Syst Rev. 2018 Jan 16;1(1):MR000039. doi: 10.1002/14651858.MR000039.pub2. Cochrane Database Syst Rev. 2018. PMID: 29372930 Free PMC article.
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
-
Uterotonic agents for preventing postpartum haemorrhage: a network meta-analysis.Cochrane Database Syst Rev. 2018 Apr 25;4(4):CD011689. doi: 10.1002/14651858.CD011689.pub2. Cochrane Database Syst Rev. 2018. Update in: Cochrane Database Syst Rev. 2018 Dec 19;12:CD011689. doi: 10.1002/14651858.CD011689.pub3. PMID: 29693726 Free PMC article. Updated.
-
Vaccines for preventing influenza in healthy children.Cochrane Database Syst Rev. 2018 Feb 1;2(2):CD004879. doi: 10.1002/14651858.CD004879.pub5. Cochrane Database Syst Rev. 2018. PMID: 29388195 Free PMC article.
References
-
- Shu B., Kirby M.K., Davis W.G., Warnes C., Liddell J., Liu J., Wu K., Hassell N., Benitez A.J., Wilson M.M., et al. Multiplex real-time reverse transcription PCR for influenza A virus, influenza B virus, and severe acute respiratory syndrome coronavirus 2. Emerg. Infect. Dis. 2021;27:1821–1830. doi: 10.3201/eid2707.210462. - DOI - PMC - PubMed
-
- Hu J.X., Zhu L.B., Wu S.T., Ding S.N. Rapid and Sensitive Detection of Influenza B Virus Employing Nanocomposite Spheres Based on Ag-Doped ZnIn2S4 Quantum Dots. Chemosensors. 2024;12:68. doi: 10.3390/chemosensors12040068. - DOI
-
- Sakurai A., Takayama K., Nomura N., Kajiwara N., Okamatsu M., Yamamoto N., Tamura T., Yamada J., Hashimoto M., Sakoda Y., et al. Fluorescent Immunochromatography for Rapid and Sensitive Typing of Seasonal Influenza Viruses. PLoS ONE. 2015;10:e0116715. doi: 10.1371/journal.pone.0116715. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

