A New Fluorescence Band of Anthocyanins as a Simple Oxidation Biomarker of Food Products
- PMID: 40572476
- PMCID: PMC12196198
- DOI: 10.3390/molecules30122510
A New Fluorescence Band of Anthocyanins as a Simple Oxidation Biomarker of Food Products
Abstract
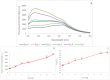
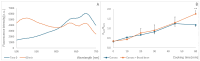
The formation of a new fluorescence band of anthocyanidins and anthocyanidins, centered at about 530 nm (excitation at 460-470 nm), is proposed as a simple indicator of food oxidation. This fluorescence band appeared and increased progressively during the incubation of blueberry juice under aerobic conditions and the cooking of blueberry homogenate and black carrot. The same effect was observed upon the addition of delphinidin to rapeseed oil subjected to simulated frying. A ratiometric parameter (ratio of the fluorescence intensity at the maximum of the new band to the fluorescence intensity of native anthocyanins/anthocyanidin) is proposed as a versatile index useful for the estimation of the oxidation of food products containing anthocyanins or supplemented with anthocyanins or anthocyanidins.
Keywords: anthocyanidins; anthocyanins; black carrot; blueberry; fluorescence; lipid peroxidation; oil; oxidation.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Li Z., Ahammed G.J. Plant stress response and adaptation via anthocyanins: A review. Plant Stress. 2023;10:100230. doi: 10.1016/j.stress.2023.100230. - DOI
-
- Silva V.O., Freitas A.A., Maçanita A.L., Quina F.H. Chemistry and photochemistry of natural plant pigments: The anthocyanins. J. Phys. Org. Chem. 2016;29:594–599. doi: 10.1002/poc.3534. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

