Enantiomerically Pure ansa- η5-Complexes of Transition Metals as an Effective Tool for Chirality Transfer
- PMID: 40572477
- PMCID: PMC12195769
- DOI: 10.3390/molecules30122511
Enantiomerically Pure ansa- η5-Complexes of Transition Metals as an Effective Tool for Chirality Transfer
Abstract
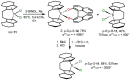
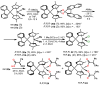
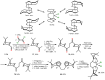
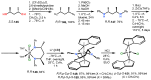
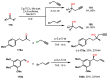
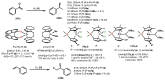
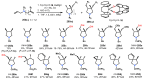
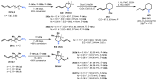
Chiral ansa-η5-complexes of transition metals have shown remarkable efficacy in organometallic synthesis and catalysis. Additionally, enantiomerically pure ansa-complexes hold promise for the development of novel chiral materials and pharmaceuticals. The discovery and synthesis of a diverse range of group IVB and IIIB metal complexes represents a significant milestone in the advancement of stereoselective catalytic methods for constructing metal-C, C-C, C-H, and C-heteroatom bonds. The synthesis of enantiomerically pure metallocenes can be accomplished through several strategies: utilizing optically active precursors of η5-ligands, separation of diastereomers of complexes with enantiomerically pure agents, and synthesis via the stereocontrolled reactions of enantiomerically pure σ-complexes with prochiral anions of η5-ligands. This review focuses on the analysis of various nuances of the synthesis of enantiomerically pure ansa-η5-complexes of titanium and lanthanum families. Their applicability as effective catalysts in asymmetric carbomagnesiation, carbo- and cycloalumination, oligo- and polymerization, Diels-Alder cycloaddition, reactions of zirconaaziridines, cyclization, hydrosilylation, hydrogenation, hydroamination, and other processes are highlighted as well.
Keywords: ansa-metallocenes; asymmetric synthesis; enantiomerically pure complexes; reaction mechanisms; stereoselectivity.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





















































































Similar articles
-
Assessing the comparative effects of interventions in COPD: a tutorial on network meta-analysis for clinicians.Respir Res. 2024 Dec 21;25(1):438. doi: 10.1186/s12931-024-03056-x. Respir Res. 2024. PMID: 39709425 Free PMC article. Review.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
-
The effectiveness of interventions to meet family needs of critically ill patients in an adult intensive care unit: a systematic review update.JBI Database System Rev Implement Rep. 2016 Mar;14(3):181-234. doi: 10.11124/JBISRIR-2016-2477. JBI Database System Rev Implement Rep. 2016. PMID: 27532144
-
Health professionals' experience of teamwork education in acute hospital settings: a systematic review of qualitative literature.JBI Database System Rev Implement Rep. 2016 Apr;14(4):96-137. doi: 10.11124/JBISRIR-2016-1843. JBI Database System Rev Implement Rep. 2016. PMID: 27532314
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
References
-
- Marek I. Titanium and Zirconium in Organic Synthesis. Wiley-VCH; Weinheim, Germany: 2002. p. 537.
-
- Takahashi T. Metallocenes in Regio- and Stereoselective Synthesis. Springer; Berlin/Heidelberg, Germany: 2005. p. 244.
-
- Zohuri G.H., Albahily K., Schwerdtfeger E.D., Miller S.A. 3.21—Metallocene Alkene Polymerization Catalysts. In: Moeller M., Matyjaszewski K., editors. Polymer Science: A Comprehensive Reference. Volume 3. Elsevier Science; Amsterdam, The Netherlands: 2012. pp. 673–697. - DOI
-
- Vaughan A., Davis D.S., Hagadorn J.R., Salwani M.S. Industrial Catalysts for Alkene Polymerization. Ref. Modul. Mater. Sci. Mater. Eng. 2017:1–18. doi: 10.1016/B978-0-12-803581-8.10252-8. - DOI
-
- Kaminsky W. Discovery of Methylaluminoxane as Cocatalyst for Olefin Polymerization. Macromolecules. 2012;45:3289–3297. doi: 10.1021/ma202453u. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

