Engineering and Exploiting Immobilized Peptide Organocatalysts for Modern Synthesis
- PMID: 40572483
- PMCID: PMC12196377
- DOI: 10.3390/molecules30122517
Engineering and Exploiting Immobilized Peptide Organocatalysts for Modern Synthesis
Abstract
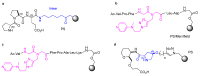
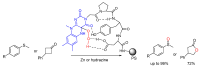
Short- and medium-sized peptides have long been used as effective and versatile organocatalysts. In the early 80s, Inoue used diketopiperazines in the Strecker reaction, while Juliá and Colonna reported the epoxidation of chalcone catalyzed by poly-L-Ala. Since then, a variety of peptide-catalyzed reactions have been described. However, peptide synthesis typically implicates the use of toxic reagents and generates wastes; therefore, peptide recycling is expected to significantly improve the overall sustainability of the process. Easy recovery and recycling of peptide catalysts can be expediently attained by covalent binding, inclusion, or adsorption. In addition, immobilization can significantly accelerate the screening of new peptide catalysts. For these reasons, diverse supports have been tested, including natural or synthetic polymers, porous polymeric networks, inorganic porous materials, organic-inorganic hybrid materials, and finally metal-organic frame-works.
Keywords: PEG; absorption; asymmetric catalysis; green chemistry; peptide organocatalyst; polystyrene; silica; β-turn.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



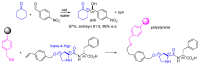







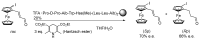
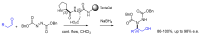
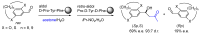
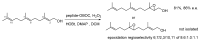
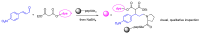
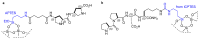

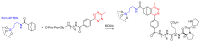


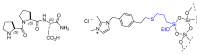


Similar articles
-
Assessing the comparative effects of interventions in COPD: a tutorial on network meta-analysis for clinicians.Respir Res. 2024 Dec 21;25(1):438. doi: 10.1186/s12931-024-03056-x. Respir Res. 2024. PMID: 39709425 Free PMC article. Review.
-
Behavioral interventions to reduce risk for sexual transmission of HIV among men who have sex with men.Cochrane Database Syst Rev. 2008 Jul 16;(3):CD001230. doi: 10.1002/14651858.CD001230.pub2. Cochrane Database Syst Rev. 2008. PMID: 18646068
-
Interventions to reduce harm from continued tobacco use.Cochrane Database Syst Rev. 2016 Oct 13;10(10):CD005231. doi: 10.1002/14651858.CD005231.pub3. Cochrane Database Syst Rev. 2016. PMID: 27734465 Free PMC article.
-
A systematic overview of chemotherapy effects in colorectal cancer.Acta Oncol. 2001;40(2-3):282-308. doi: 10.1080/02841860151116367. Acta Oncol. 2001. PMID: 11441937
-
Comparison of the effectiveness of inhaler devices in asthma and chronic obstructive airways disease: a systematic review of the literature.Health Technol Assess. 2001;5(26):1-149. doi: 10.3310/hta5260. Health Technol Assess. 2001. PMID: 11701099
References
-
- Kuster T.H.R., Schnitzer T. Peptide catalysis: Trends and opportunities. Chem. Catalysis. 2025;5:101339. doi: 10.1016/j.checat.2025.101339. - DOI
-
- Juliá S., Masana J., Vega J.C. “Synthetic enzymes”. Highly stereoselective epoxidation of chalcone in a triphasic toluene-water-poly[(S)-alanine] system. Angew. Chem. Int. Ed. 1980;19:929–931. doi: 10.1002/anie.198009291. - DOI
-
- De Marco R., Tolomelli A., Greco A., Gentilucci L. Controlled solid phase peptide bond formation using N-carboxyanhydrides and PEG resins in water. ACS Sustain. Chem. 2013;1:566–569. doi: 10.1021/sc400058r. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

